Heat exchanged in a cyclic process
One mole of ideal monoatomic gas is taken round the cyclic process . The following is a schematic graph of a process .
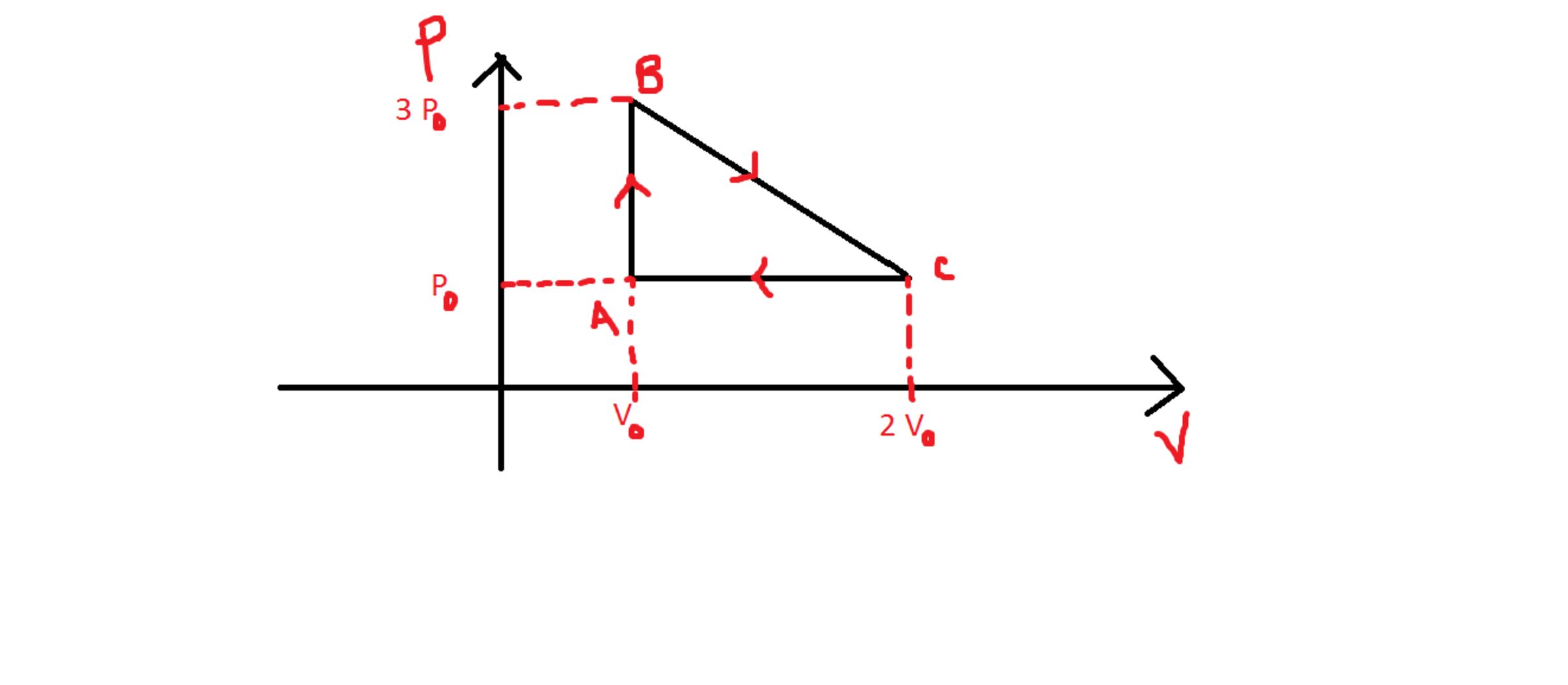
Find the heat exchange in the process .
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
In an isochoric process,
d Q = d U + d W
( here d is short for delta not differential)
But d U = u C v d T = C v d T
(u is short for mu - no. of moles =1 )
Also d W = P d V = 0 since d V = 0 for an isochoric process.
Hence d Q = d U = C v d T
From Ideal gas equation, we get,
d T = d P V / R ( since u =1 )
Substituting,
d Q = C v d P V / R = 3 / 2 R ∗ 2 P ∗ V / R = 3 P V
( because for monoatomic ideal gases gamma = C p / C v = 5 / 3 and also C p - C v = R from which we get C p = 3 / 2 )