600,000 Points Problem
If I 1 = ∫ 0 ∞ x s i n x d x and I 2 = ∫ 0 ∞ x s i n 3 x d x
Now say k be, k = I 2 I 1 .
Let following summation be S . S = n = 1 ∑ ∞ ( 7 n − 6 n ) . ( 7 n + 1 − 6 n + 1 ) 7 n . 6 n
If a reaction from reactant A to product B have 1st order forward rate constant K and first order backward rate constant(from B back to A) K − 1 .
A B
Find the concentration of B(in M)= j after long time, if the initial concentration [ A ] o = 1 M and K = 1 s e c − 1 and K − 1 = 2 s e c − 1 . (Take initial concentration of B to be 0)
Input k × j × S
The answer is 4.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
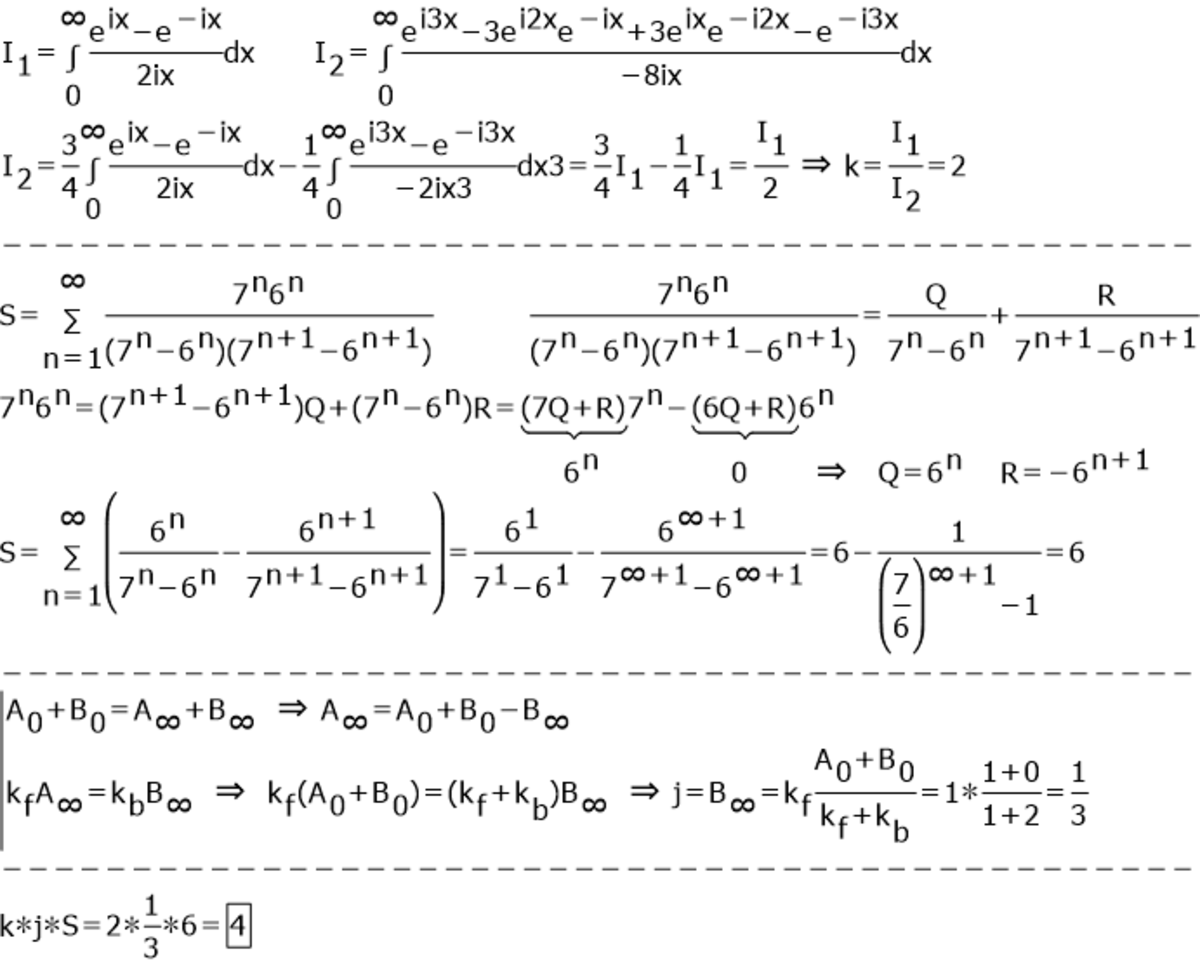
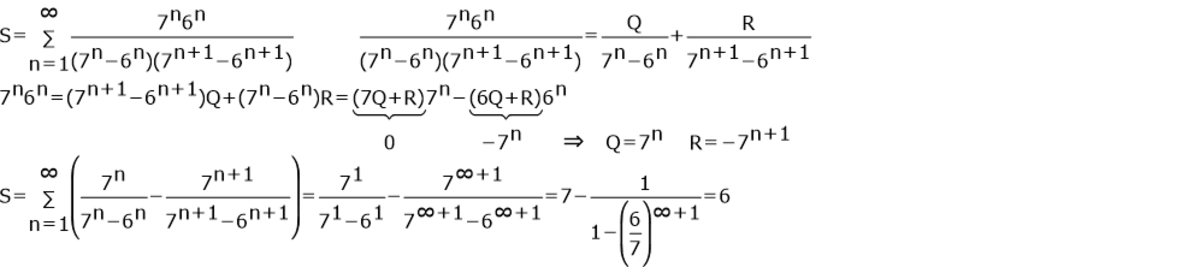
To begin with, I 1 = ∫ 0 ∞ x sin x d x = ∫ 0 ∞ x sin 3 x d x = ∫ 0 ∞ x 3 sin x − 4 sin 3 x d x = 3 I 1 − 4 I 2 so that I 1 = 2 I 2 , and hence k = 2 .
Since ( 7 n − 6 n ) ( 7 n + 1 − 6 n + 1 ) 6 n × 7 n = 7 n − 6 n 6 n − 7 n + 1 − 6 n + 1 6 n + 1 we deduce that n = 1 ∑ N ( 7 n − 6 n ) ( 7 n + 1 − 6 n + 1 ) 6 n × 7 n = 6 − 7 N + 1 − 6 N + 1 6 N + 1 for any N , and hence S = 6 .
Finally, the differential equation − d t d [ A ] = [ A ] − 2 ( 1 − [ A ] ) = 3 [ A ] − 2 or d t d [ A ] + 3 [ A ] = 2 with the initial condition [ A ] ( 0 ) = 1 , has the solution [ A ] = 3 2 + 3 1 e − 3 t and so the concentration of B is [ B ] = 1 − [ A ] = 3 1 − 3 1 e − 3 t which converges to j = 3 1 as t → ∞ .
Thus k × j × S = 2 × 6 × 3 1 = 4 .