A chemistry problem by Gandhali Rangan
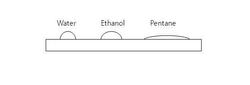 The above figure shows the cross sectional shape of water, ethanol and pentane when you drop an equal volume of each liquid on a glass plate. Which of the following orders regarding
is correct?
The above figure shows the cross sectional shape of water, ethanol and pentane when you drop an equal volume of each liquid on a glass plate. Which of the following orders regarding
is correct?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
The difference in the shape is due to the intermolecular attraction and
Δ H vapor is proportional to the strength of these intermolecular interactions.