A chemistry problem
Chemistry
Level
2
Which has a higher boiling point?
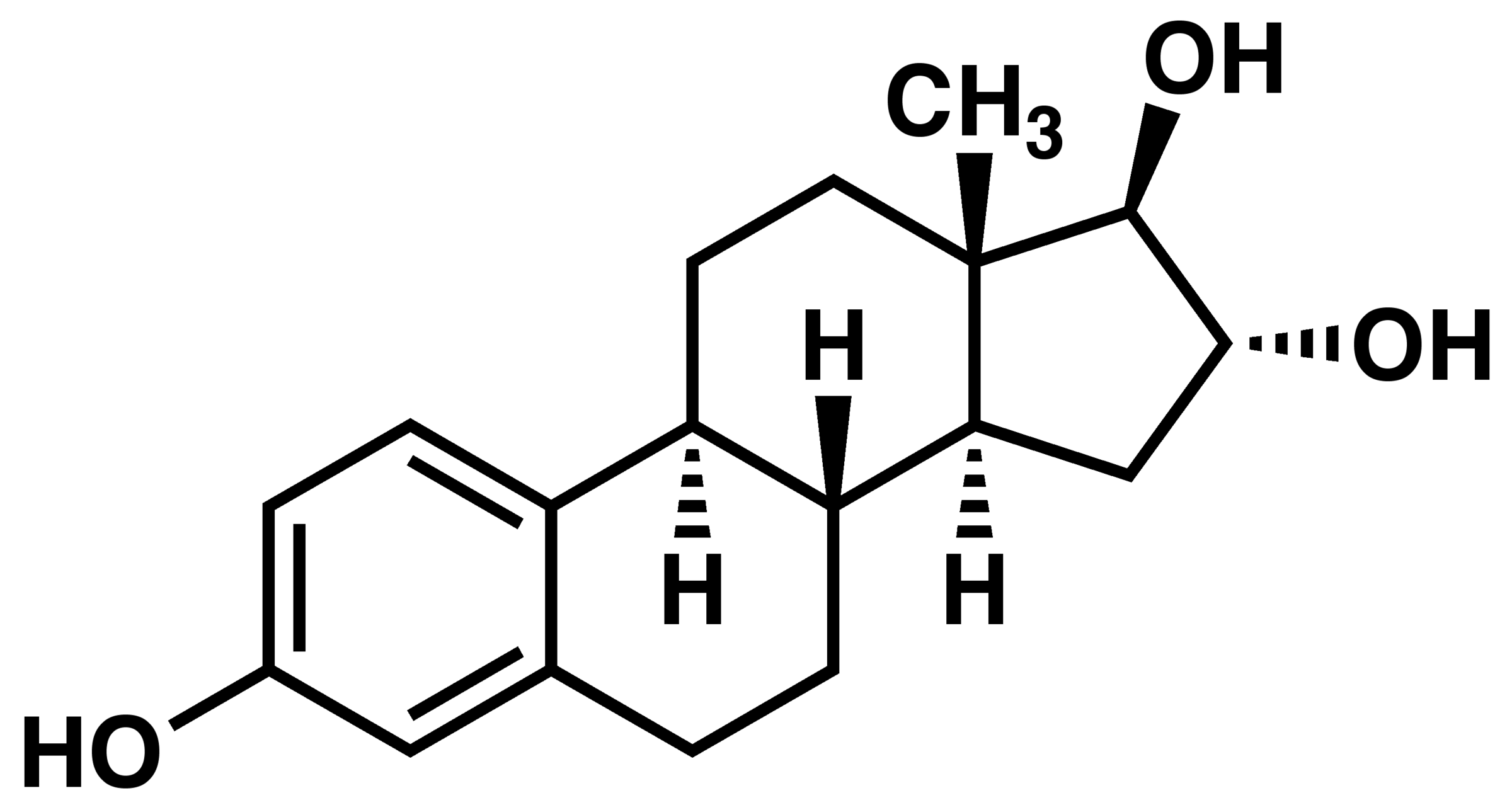 Estrogen.
Estrogen.
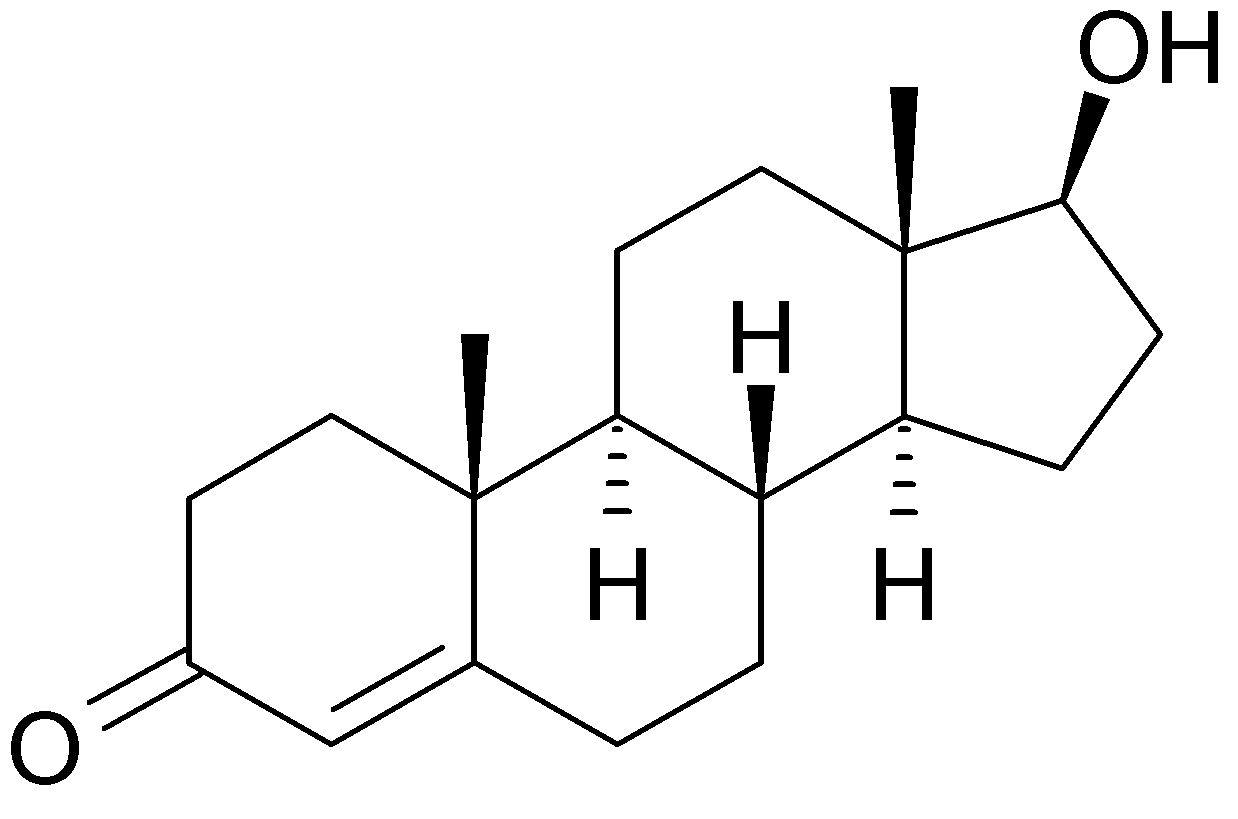
Testosterone.
Estrogen
Testosterone
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Here's my non-chemist reasoning (forgive me if it's not exactly right):
1) Things with OH are alcohols (or alcohol-like)
2) Alcohols tend to be quite volatile (tending to boil / evaporate at low temperatures)
3) Estrogen has more (OH)'s than testosterone does, suggesting that it is the more "alcoholic" of the two.
4) Therefore, estrogen is more apt to boil than testosterone is.
5) Therefore, testosterone probably has the higher boiling point (needing more internal energy in order to boil).