Don't worry about Stereochemistry
In 2004 a compound with the formula was isolated form the aerial parts of Coriaria nepalensis , an important component in Chinese herbal medicine. It was named Corianlactone . The following scheme looks into the synthesis of a mixture of Corianlactone and its diastereomers:
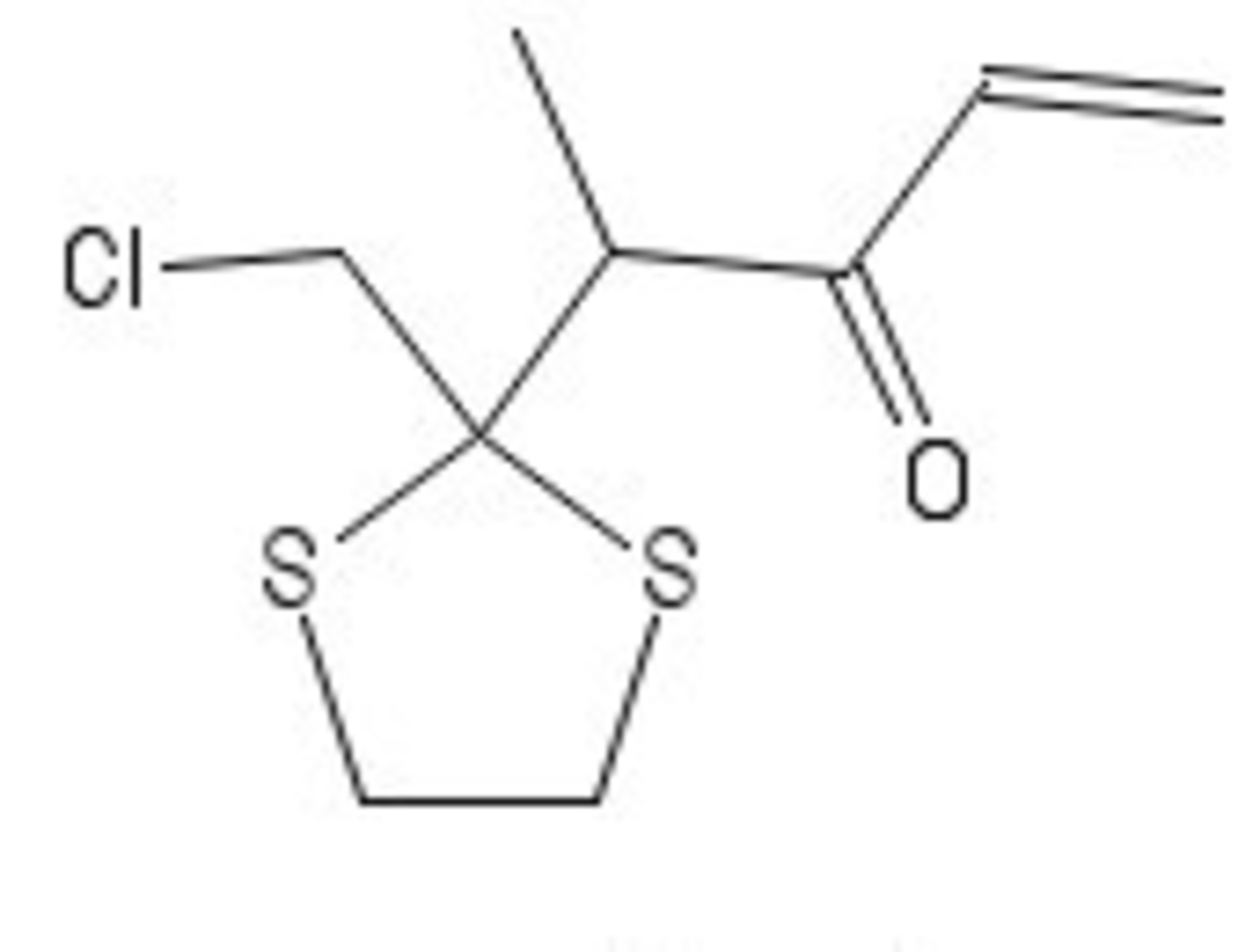
We start with 2-Iodo 5-methylfuran and the above compound. Key steps are cycloadditons
- They react in presence of Palladium(II) acetate to form A
- Compound A reacts with the intermediate whose formula is (that is formed from Triphenylphosphine) to form compound B
- B reacts with to form C whose formula is
- C is mildly heated in presence of a base to form a compound D
- D reacts with in presence of Iodine and Methyl Chloride to form a E whose formula is
- E is heated in presence of an intermediate that is formed when acetyl chloride is heated with a base to give us F
- F reacts with an excess of Meta Chloro Per Benzoic Acid and the resultant compound then reacts with to give us the required mixture.
What is the number of rings in compound D ?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
We start from C whose chemical formula is known to us. The reaction of B to C is a simple dithiane removal and A to B is a Wittig reaction involving Triphenyl methyl phosphonium ylide (this is deduced from the formula). This tells us that the A possesses a chemical formula C 1 4 H 1 7 O 2 S 2 C l implying a coupling reaction must occur :
The structures of C and B are easily found after this :
We now shift our focus to E which can be thought of as a dithiane protected D . Knowing this, compound D 's chemical formula is found to be C 1 3 H 1 4 O 2 . Now it becomes apparent that during the conversion of C to D that a loss of H C l must occur. The reaction conditions support a cyclo-addition between the furan ring and the de-hydrochlorinated moiety.
The heating of acetyl chloride in presence of a base generates a ketene that reacts with E to form F :
The next set of reactions are the Baeyer-villager oxidation and Epoxidation, followed by dithiane removal to generate a racemic mixture containing Corianlactone :
D contains two five membered rings and one six membered ring and hence the answer is 3