Shifts and Signals
An extension to Teleanu Florin's problem (for context you might want to go through his problem)
This is the compound that was investigated in the problem, we call it compound X :
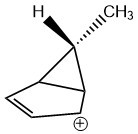
Let all the hydrogen atoms bonded to the carbon atoms in the cyclopentane ring be denoted as Ring Hydrogens or and the remaining hydrogen on the top of the cyclopropane ring be called the Axial Hydrogen or . The hydrogen atoms present in the axial methyl group are denoted as .
Let's say we have some kind of reaction that allows us to remove a atom and replace it with a deuterium ( ) without changing the rest of the molecule.
We now turn to H-NMR spectra of 3 compounds:
- Compound X
- Compound Y (one is replaced)
- Compound Z (two are replaced)
(All compounds are placed in solutions that are above 390K)
We now define 2 quantities and . is the number of H-NMR signals obtained from compound Y and is the number of H-NMR signals obtained from compound Z
What is the value - ?
Bonus :
Devise a reaction that can replace with
The answer is 0.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
As Teleanu Florin points out in his solution, the HOMO (Highest Occupied Molecular Orbital) of the C 4 and C 5 bond and the LUMO (Lowest Occupied Molecular Orbital) of C 1 ie., the empty p-orbital are involved in a [1 4] electrocyclic shift. This is illustrated below:
However this process repeats infinitely, hence there exist in 5 canonical forms. These 5 forms are shown below:
These canonical forms create a resonance hybrid . This explains why the H-NMR spectra of the compound never changes even though an infinite number of [1 4] shifts occur while observing it. (It looks like a capped pentagon)
Using the yet to be determined reaction mechanism (YDRM) we generate compound Y :
Each signal in a H-NMR represents a different chemical environment that hydrogen can be placed in.It is important to note that deuterium atoms are invisible to the H-NMR spectra.
Hence in compound Y we see 4 unique environments:
Since four unique environments exist ⟹ α = 4
We use YDRM again to generate compound Z . We now encounter a problem, the new deuterium atom can either be placed adjacent to the first deuterium atom or be placed one atom away from it.
CASE ONE : Deuterium atoms are adjacent to each other
In compound Z 1 there exist:
Since four unique environments exist, β 1 = 4
CASE TWO : Deuterium atoms are separated by one H r
In compound Z 2 there exist:
Again four unique environments exist, β 2 = 4
Since β 1 = β 2 ⟹ β = 4
So the answer is α − β = 0
Side note:
If you can design a reaction mechanism that does what YDRM is designed to do, please let me know. I still haven't been able to design one.