Thermodynamics
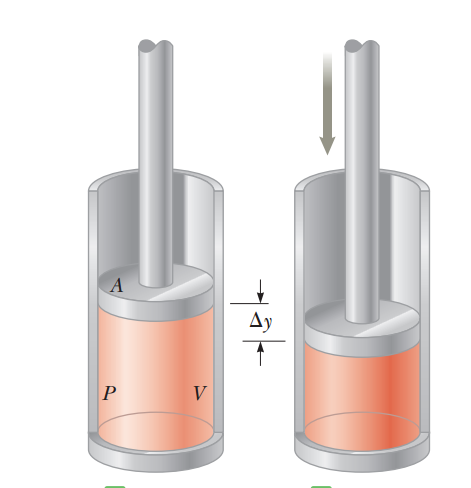
A vessel containing helium { considering it to be an ideal } which expands at constant pressure when of heat is supplied to it. What will be the variation of internal energy ; work done by gas respectively
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
helium is a monoatomic gas so degree of freedom(f) = 3 C V = 2 f R C V = 2 3 R By mayor's formula C p = C V + R Δ Q = n C p Δ T Δ U = n C V Δ T Δ Q Δ U = C P C V = 5 3 ∴ Δ U = 1 5 × 5 3 Δ W = Δ Q − Δ U = 1 5 − 9 = 6 k J