pressure pressure on the container which is correct answer
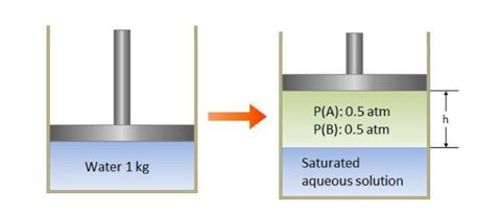 The above left is a cylinder containing
kg of water at
°C. Then gases A and B nonreactive to each other are added. After enough time passes, as the picture on the right shows, the height h is stabilized with partial pressures of both gases being
atm. The solubilities of gas A and B at 0°C and 1 atm are
The above left is a cylinder containing
kg of water at
°C. Then gases A and B nonreactive to each other are added. After enough time passes, as the picture on the right shows, the height h is stabilized with partial pressures of both gases being
atm. The solubilities of gas A and B at 0°C and 1 atm are
of water
of water
respectively. If the formula weight of B is twice as much as that of A and the mass of the piston is negligible, which of the following statements is correct?
a) The dissolved weight of gas B is twice as much as that of gas A. b) The number of moles of gas A and B, when added initially, were not the same. c) The molalities of gas A and B, after being dissolved in the water, are the same.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Id they exert the same pressure, the number of melecules is the same