A duel of stability
Chemistry
Level
1
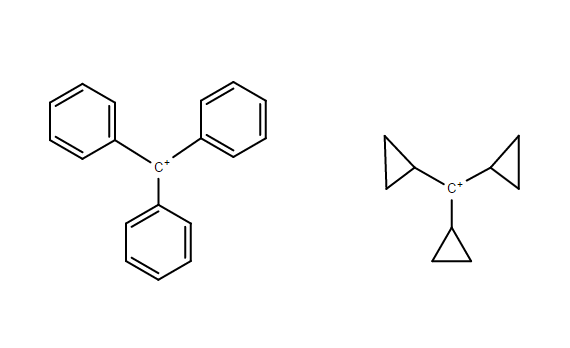
Which one is the more stable carbocation out of the two?
Tertiary benzyl carbocation
Tricyclopropyl methyl carbocation
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Strain plays a key role in determining the stability order.
The Tricyclopropyl methyl carbocation is greatly benefited by angle strain. The bent bond effect greatly stabilizes the cation by providing a large pseudo - pi system that involves the electrons of the "bent bonds" of the cyclopropyl rings
Conversely, the Triphenyl methyl carbocation suffers form steric strain. The large phenyl rings must rotate out of the plane to overcome this (as shown below). The angle of rotation does not cut off the empty p-orbital from the pi system instead it just reduces the resonance contribution as the orbital overlap is not optimal.
It is due to these two factors that the Tricyclopropyl methyl carbocation is more stable when compared to the Triphenyl methyl carbocation (Trityl carbocation)
Image taken from Wikipedia.