A mixture of Organic and Physical Chemistry
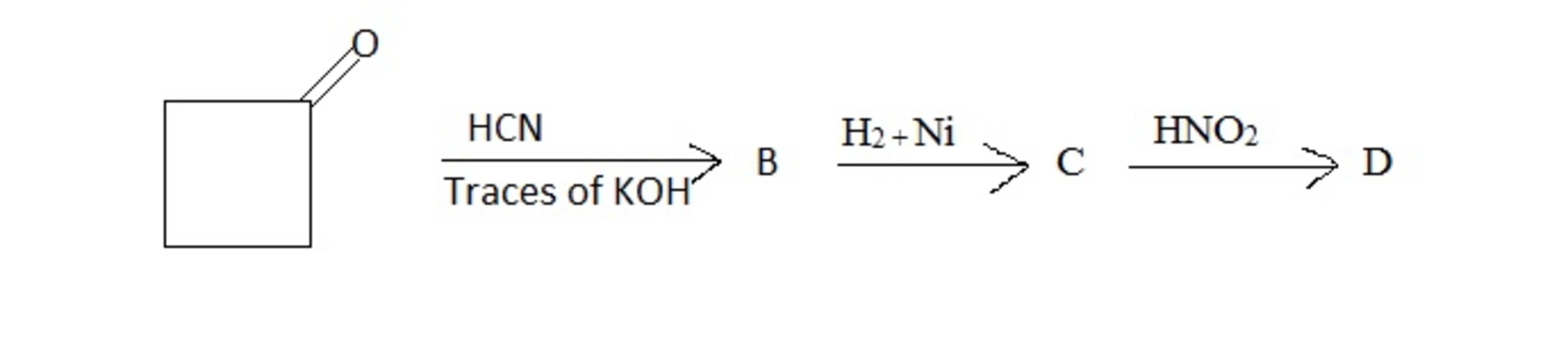
The number of statements given below which are correct with respect to the given picture above are
-
In the formation of from ring expansion takes place.
-
The product is cyclopentanone.
-
The product is - unsaturated cyclopentanone.
-
The conversion of to can be carried out with
Evaluate
At a given temperature, the total vapour pressure (in torr) of a mixture of volatile components and is given by
Evaluate the magnitude of difference in vapour pressures (in torr) of pure components and
The answer is 75.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
First of all I'm sorry since I can't draw the structures here .
The only the second statement is wrong , hence n = 3 .
Hence j = 7 5 , k = 5 ! = 1 2 0 .
We all know that P A and P B have been calculated when they are in pure conditions
Let's consider that time when only component B was present in the solution i.e. X B = 1 and P T o t a l = P B ° , P T o t a l = 1 2 0 − 7 5 ⋅ X B ∴ P T o t a l = 1 2 0 − 7 5 = 4 5
Let's consider that time when only component A is present in the solution i.e. X B = 0 and P T o t a l = P A ° , P T o t a l = 1 2 0 − 7 5 ⋅ X B ∴ P T o t a l = 1 2 0 − 0 = 1 2 0
Therefore our answer is ∣ 1 2 0 − 4 5 ∣ = 7 5 .
NOTE : P T o t a l is independent of X A .