Titanic sink
 RMS Titanic was a British passenger liner that sank in the North Atlantic Ocean on
April
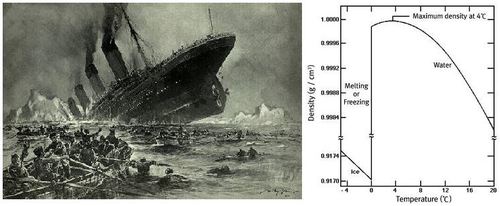
after colliding with an iceberg during her maiden voyage from Southampton, UK to New York City, US. The solid form of most substances is denser than the liquid phase; thus, a block of most solids will sink in the liquid. However, a block of ice floats in liquid water because ice is less dense. (The above right is the density of ice and water as a function of temperature.) Which of following is relevant to this phenomenon?
RMS Titanic was a British passenger liner that sank in the North Atlantic Ocean on
April
after colliding with an iceberg during her maiden voyage from Southampton, UK to New York City, US. The solid form of most substances is denser than the liquid phase; thus, a block of most solids will sink in the liquid. However, a block of ice floats in liquid water because ice is less dense. (The above right is the density of ice and water as a function of temperature.) Which of following is relevant to this phenomenon?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Hydrogen bonding in ice causes it to have air spaces between molecules...thus decreasing density....