A Radioactive Problem
 Plutonium-239 has been used as a power source for heart pacemakers. The chemical reaction taking place is
Plutonium-239 has been used as a power source for heart pacemakers. The chemical reaction taking place is
9 4 2 3 9 P u ⟶ 2 4 H e + 9 2 2 3 5 U
Let the energy obtained from the following decay of 2 1 5 mg of plutonium-239 be A J .
Let B g be the minimum mass of Zn that would be needed for the standard voltaic cell
Z n ∣ Z n X 2 + ∣ ∣ C u X 2 + ∣ C u
to obtain the same amount of electrical energy.
What is the value of 1 0 0 0 B A when rounded to the nearest integer?
Details and Assumptions
- Standard cell potential: E cell ∘ = 1 . 1 V .
- Atomic masses: H e = 4 . 0 0 1 5 u , U = 2 3 4 . 9 9 3 5 u , P u = 2 3 9 . 0 0 0 6 u .
You can try more of my Questions here .
The answer is 3.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
5 solutions
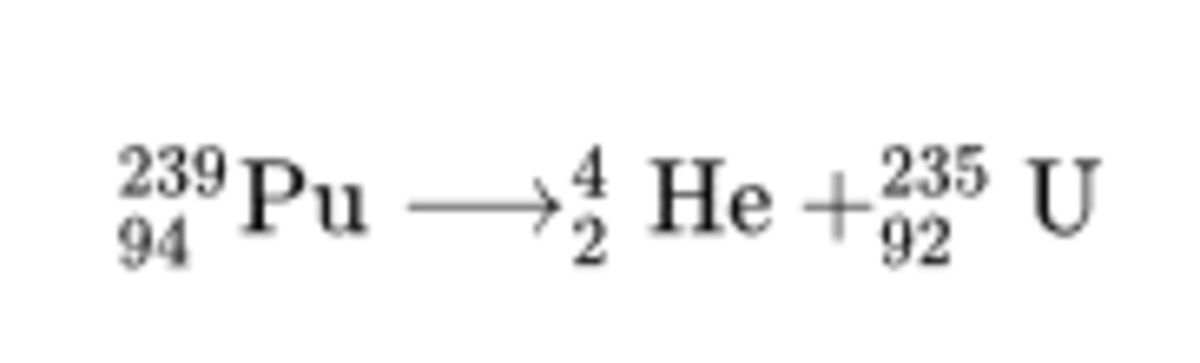 delta m = ( mass Pu) - (mass U + mass H)
delta m = (239.0006) - (234.9935 + 4.0015)
delta m = 0.0056 u/atom
delta m = ( mass Pu) - (mass U + mass H)
delta m = (239.0006) - (234.9935 + 4.0015)
delta m = 0.0056 u/atom
/u = 1.66x10^-24 gram/u mass defeat = 0.0056 u/atom x 1,66e27 kg/u mass defeat = 8.96e30 kg/atom
mol plutonium = 0.215 gram / 239.0006 g/mol mol plutonium = 0.001 mol
atom plutonium = mol x B.Avogadro atom plutonium = 0.001 x 6.02e23 atom plutonium = 602000000000000000000 atom
mass total = 602000000000000000000 atom x 8.96e30 kg/atom mass total = 5.39e-9 kg
E = mc^2 E = 5.39e-9 kg x (3e8)^2 E = 480600000 joule (A)
to obtain the same amount of electrical energy = 480600000 joule 1,10 volt = 1,10 J/C mass Zn = 480600000 joule x (1C / 1,1 Joule) x (1 mol e / 96500 C) (1 mol Zn / 2mol e) (65.39 gram mol Zn / 1 mol Zn) mass Zn = 148028.443 gram (B)
A/B = 480600000 / 148028.443 = 3246.673
The energy obtained from the nuclear reaction X 9 4 2 3 9 X 2 9 4 2 2 3 9 P u X 2 4 X 2 2 2 4 H e + X 9 2 2 3 5 X 2 9 2 2 2 3 5 U is from the missing mass of the reaction. The energy available from one atomic mass unit u by Einstein's equation E = m c 2 (where c is the speed of light in free space) is E = u c 2 J. The missing mass due to the decay of a Pu-239 atom is A r ( P u ) − A r ( H e ) − A r ( U ) = 2 3 9 . 0 0 0 6 − 4 . 0 0 1 5 − 2 3 4 . 9 9 3 5 = 0 . 0 0 5 6 u . The energy obtained from the delay of 1 mol of Pu-239 is E m o l = 0 . 0 0 5 6 N A u c 2 J, where N A is the Avogadro constant. Therefore, that from 2 1 5 mg of Pu-239 is:
A = 2 3 9 . 0 0 0 6 0 . 2 1 5 × 0 . 0 0 5 6 N A u c 2 = 2 3 9 . 0 0 0 6 0 . 2 1 5 × 0 . 0 0 5 6 × 6 . 0 2 2 1 4 × 1 0 2 3 × 1 . 6 6 0 5 4 × 1 0 − 2 7 × ( 2 . 9 9 7 9 2 × 1 0 8 ) 2 = 4 . 5 2 7 6 0 × 1 0 8 J
The electrical energy available from n mol of electrons is E V = n N A e E c e l l ∘ J, where e = 1 . 6 0 2 1 8 × 1 0 − 1 9 C is the electron charge. Putting E V = A , we have n N A e E c e l l ∘ = 4 . 5 2 7 6 0 × 1 0 8 ⇒ n = N A e E c e l l ∘ 4 . 5 2 7 6 0 × 1 0 8 . Since each mol ( 6 5 . 3 8 g) of Z n gives two mol of electrons, then:
B = 2 n × 6 5 . 3 8 = 2 × 6 . 0 2 2 1 4 × 1 0 2 3 × 1 . 6 0 2 1 8 × 1 0 − 1 9 × 1 . 1 4 . 5 2 7 6 0 × 1 0 8 × 6 5 . 3 8 = 1 . 3 9 4 5 3 × 1 0 5 g
⇒ 1 0 0 0 B A = 1 . 3 9 4 5 3 × 1 0 8 4 . 5 2 7 6 0 × 1 0 8 = 3 . 2 4 6 6 8 ≈ 3
.
We do not have evidence but it is good to consider that strong force could relate to glue which has mass which turned into energy or away from weighing.
Applying notation of 1E+4 = 1 × 1 0 4 :
Before (g/ mol): 239.0006
After (g/ mol): 4.0015 + 234.9935 = 238.995
Lost of mass per mole (kg/ mol) = (239.0006 - 238.995)/ 1000 = 5.6E-06
Number of mole of 9 4 2 3 9 P u = 2 3 9 . 0 0 0 6 0 . 2 1 5 = 8.99579331600004E-04
Speed of light, c (m/ s) = 299792458
Only way available is to apply E = m c 2 = 8.99579331600004E-04 × 5.6E-06 × 299792458 2 = 4.52760886456606E+08 J = A
http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/electrochem.html
E c e l l = 1 . 1 V
Faraday constant = 96485.33289 Columbs/ mol
Number of mole of e − required = F a r a d a y c o n s t a n t × E c e l l E n e r g y = 9 6 4 8 5 . 3 3 2 8 9 × 1 . 1 4 . 5 2 7 6 0 8 8 6 4 5 6 6 0 6 E + 0 8 = 4 . 2 6 5 9 4 1 7 0 8 8 6 9 8 4 E + 0 3
Number of mole of Z n 2 + required = 2 4 . 2 6 5 9 4 1 7 0 8 8 6 9 8 4 E + 0 3 = 2 . 1 3 2 9 7 0 8 5 4 4 3 4 9 2 E + 0 3
Mass of Zn required = 2.13297085443492E+03 mol × 6 5 . 3 8 g / m o l = 1.39453634462955E+05 g = B
⌊ 1 0 0 0 B A ⌋ = ⌊ 1 . 3 9 4 5 3 6 3 4 4 6 2 9 5 5 E + 0 8 4 . 5 2 7 6 0 8 8 6 4 5 6 6 0 6 E + 0 8 ⌋ = ⌊ 3 . 2 4 6 6 7 6 8 4 8 5 4 6 9 6 ⌋ = 3 (Also equals to value rounded to the nearest integer.)
C a u t i o n : It was stated in a book that "the change in mass is extremely small and cannot be detected by even the most sensitive devices" with exact certainty but determined with facts. [Mass changed into energy should not be treated as an extreme mass.] Appreciate the ratio of mass lost to mass of zinc required for same amount of energy obtained.
Answer: 3
The problem is befooling one. We have to just get energy per gram ratio for the cell Given. Actually the A joule divided by B gram denotes x joule per gram of zinc. I think all can evaluate that.its equal to emf*Faraday charge/ eqv mass.
Δ m c 2 ⋅ n p l u t o n i u m = n F E c e l l o ⋅ n z i n c ⇒ A = ( 2 ) ( 9 6 5 0 0 ) ( 1 . 1 ) 6 5 . 4 B ⇒ 1 0 0 0 B A = ( 6 5 . 4 ) ( 1 0 0 0 ) ( 2 ) ( 9 6 5 0 0 ) ( 1 . 1 )
I don't think it can be simpler.