A rather different one!
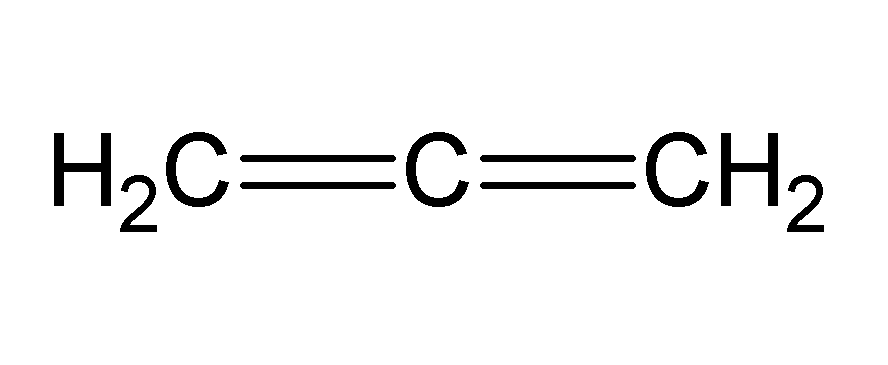
The compound shown above is one of the simplest forms of a type of compound known as allene. An allene is a hydrocarbon containing two consecutive double bonds in the parent chain.
The above compound is known as propa-1,2-diene and is majorly used as a constituent of a gas used as a fuel for specialized welding.
What is the maximum number of separate atoms in propa-1,2-diene that lie on the same plane?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
The structure above signifies that one of two pairs of hydrogen atoms connected to either of the terminal carbon lies on a different plane from the plane that contains the other five atoms. Hence, the maximum number of atoms lying on one plane is 5 .
Reason and explanation:
The main reason for such a structure is due to the different orientation of overlapping of atomic orbitals of the terminal carbon atoms with the central carbon.
We already know that carbon forms bonds by sharing the electrons in its p − shell which has three separate orbitals namely, p x , p y and p z respectively. The orientation of these atomic orbitals are parallel to the x , y and z axes respectively. Therefore, from a particular point of reference, two of these orbitals will lie on one plane and the other one will be left out.
If we consider that p x orbital of each of the three carbon atoms form σ bonds then each carbon will be left out with p y and p z orbitals for the formation of π bonds. If the central carbon atom uses its p y electrons to form π bond with one of the terminal carbon atoms then it will have to use its p z electrons to form π bond with the other carbon atom. Thus, the orientation of overlapping of atomic orbitals will be different and any element, group or substituent attached with one of the terminal carbon atoms will lie on a plane different from the one containing the three carbon atoms and the elements, groups, or substituents attached with the other terminal carbon.