A Review of Intelligence Reports

Carbenes were long thought of as useless and very unpredictable reagents. Many in the industry stated that its usefulness was negated just like its charge, the pros were equal to the cons.
However, in 1962, public perception changed and a new species of carbenes was discovered. Later in 1991, new developments turned them into miracle of the late century and early 2000s. Let us take a quick look at how we can produce such species and explore how they form strong bonds with metal atoms.
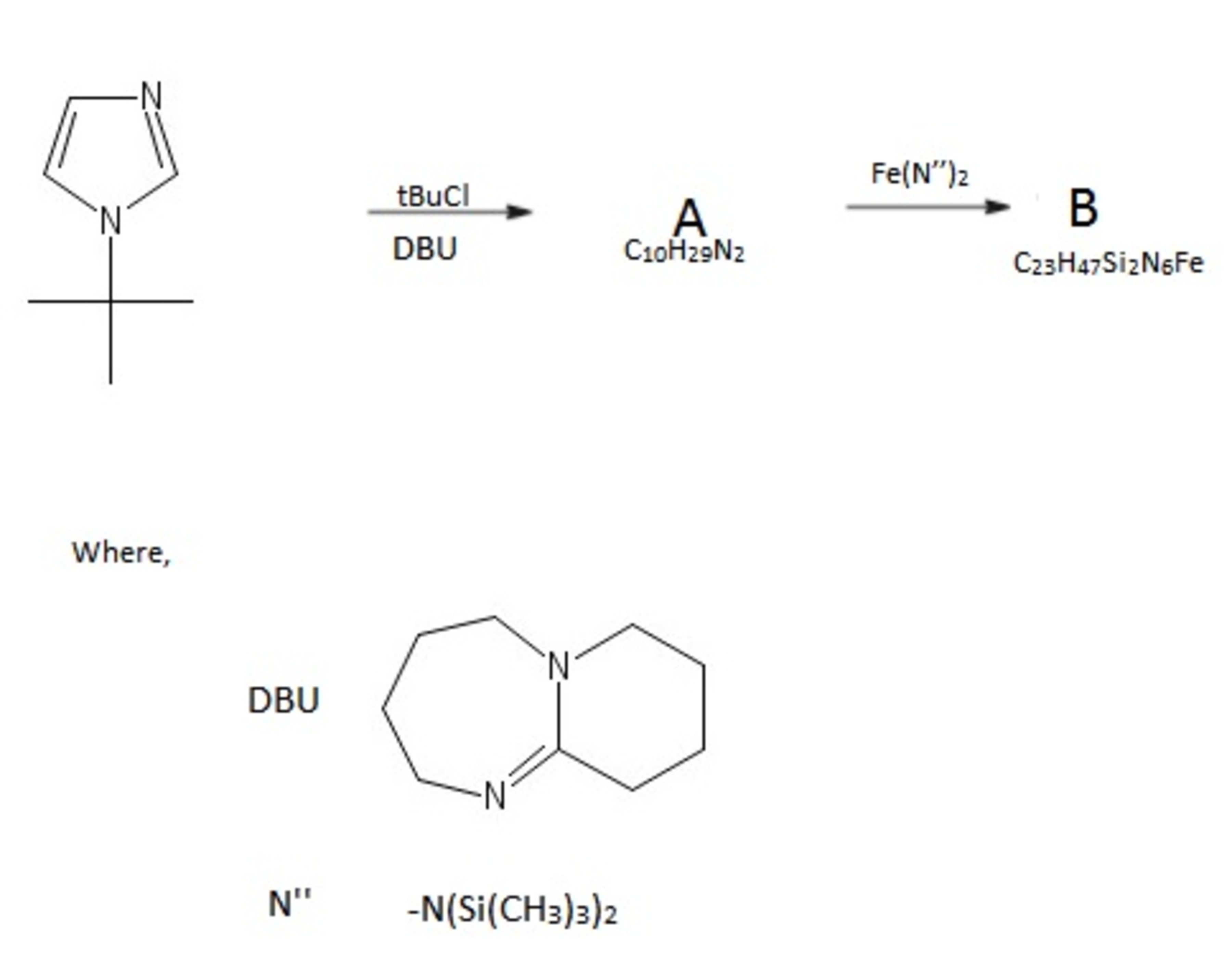
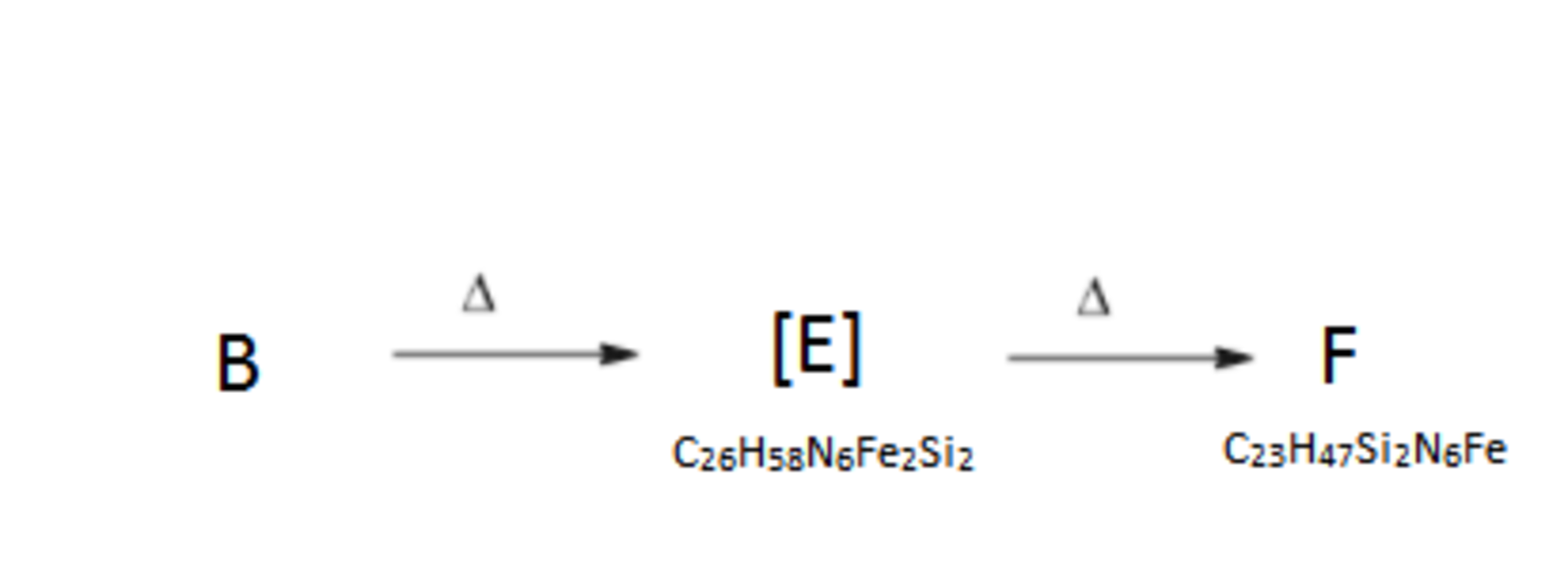
The complex B evidently suffers from steric strain and hence one would assume that heating would result in the bond breaking. Surprisingly it decomposes to produce a much more stable complex F through an intermediate E . Computational methods have shown that this intermediate possesses a centre of symmetry element.
With a basic understanding of these two interesting properties, we move on to some of new green reactions that this compound participates in:
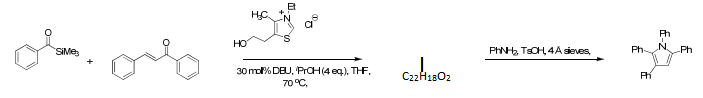
The first is a condensation reaction which generates key intermediates for heterocycle construction.
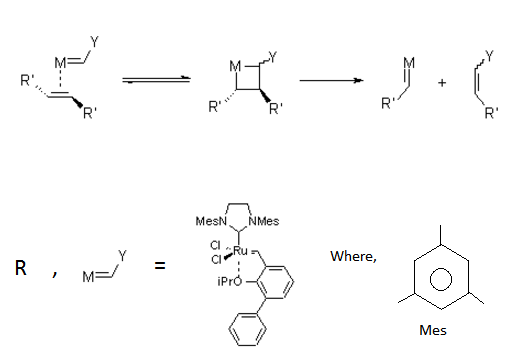
The biggest breakthrough brought about by these carbenes in the last few decades, arguably the century, is the metathesis reaction . An illustration of the mechanism is provided below : Note that R' denotes alkyl/aryl groups while R denotes the complex itself.
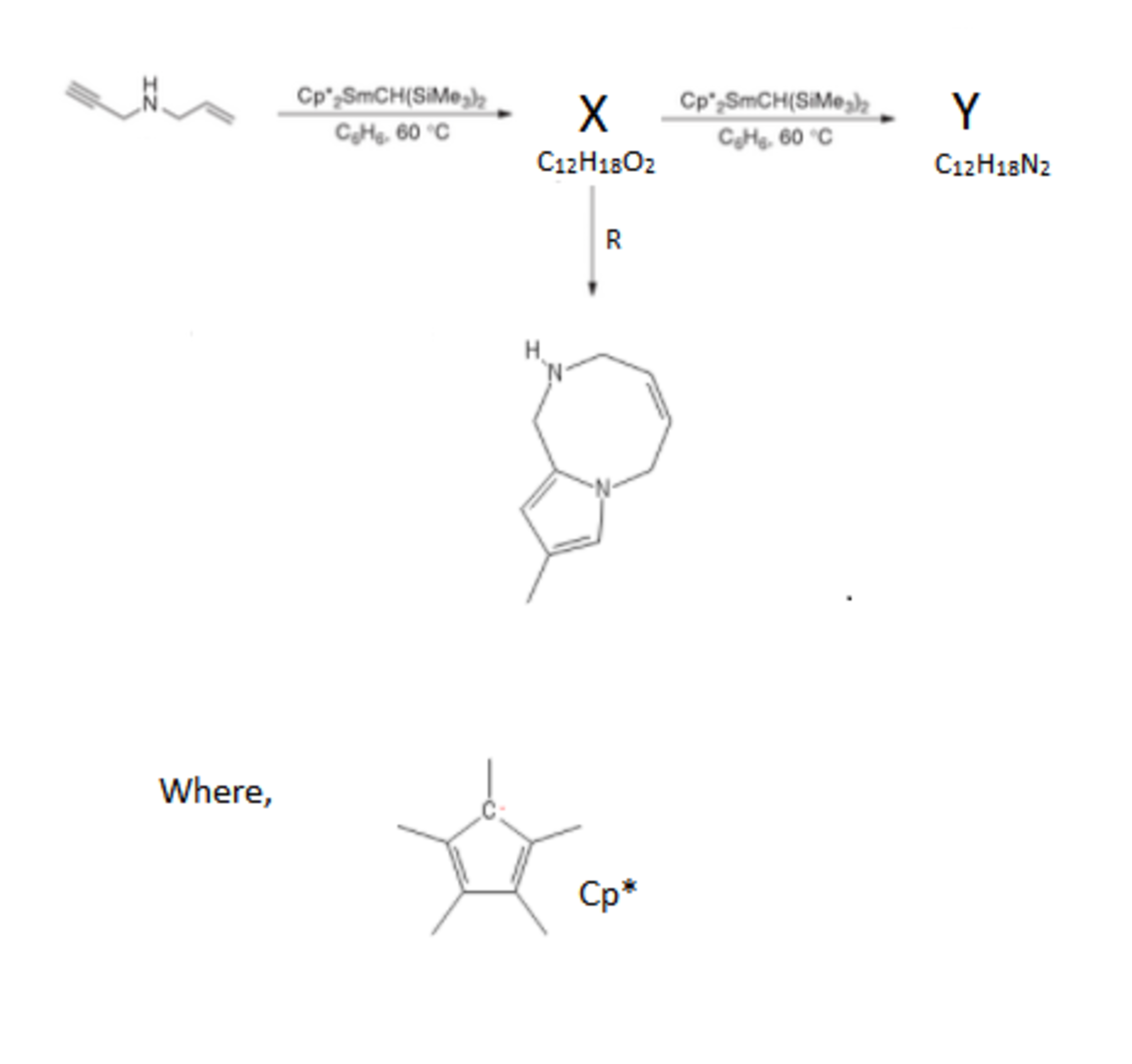
Just like how these complexes activate double bonds, another complex activates amine groups in a catalytic manner. This complex helps produce various polycyclic skeletons. Intermediate X is quite stable and only when it reacts with the complex for a longer period of time the final product Y is formed. During the catalytic cycle it is known that there are successive attacks , first by the nitrogen atom and later by the activated bond.
If denotes the number of rings in B , denote the number of rings in F , denote the number of rings in I , and denote the number of rings in Y .
Calculate the value of
BONUS
- Deduce structures of A - B , E - F , I , and X - Y
- Provide a possible catalytic cycle for the formation of Y
- Which House Intelligence Committee member was born on / / (dd/mm/yyyy)
All images belong to their respective owners.
The answer is 6.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
0 solutions
No explanations have been posted yet. Check back later!