A Vanderwal favorite

The use of the compound W in organic synthesis for a variety of alkaloids has grown in recent years. Pyridine can be thought of as the dehydrated form of W . Interestingly W cannot be formed by the hydrolysis of Pyridine. Instead it requires another reaction, illustrated in the example below:
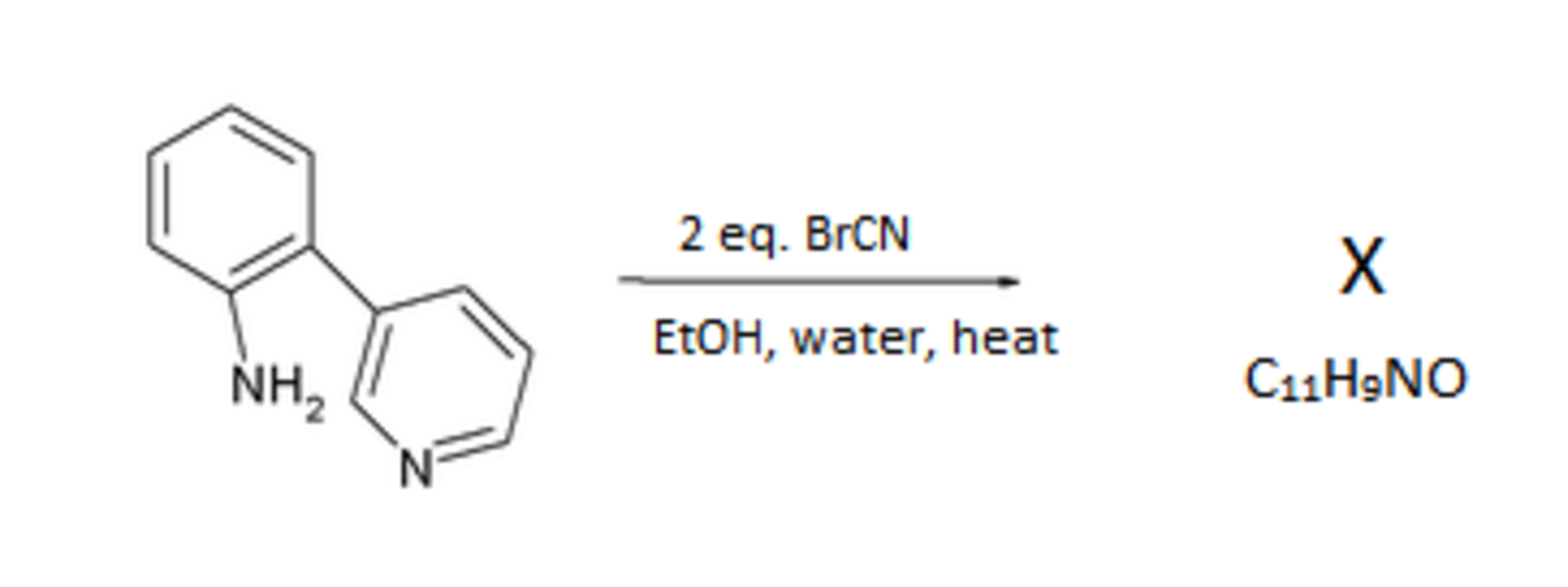
With this basic understanding of the formation of W and its derivatives, we now explore the chemistry that it contains within it. The below scheme illustrates an interesting reaction: (It is observed that Z contains an amide group and Ar denotes an aromatic group)
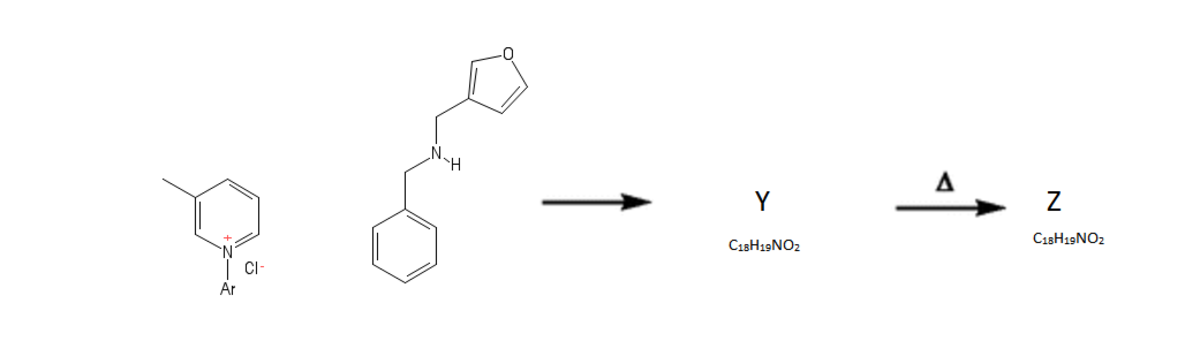
With this knowledge of the chemistry of W , we move on to a synthesis, proposed by American researchers, of a compound that can be considered as a core of an alkaloid isolated from Strychnos nux-vomica .
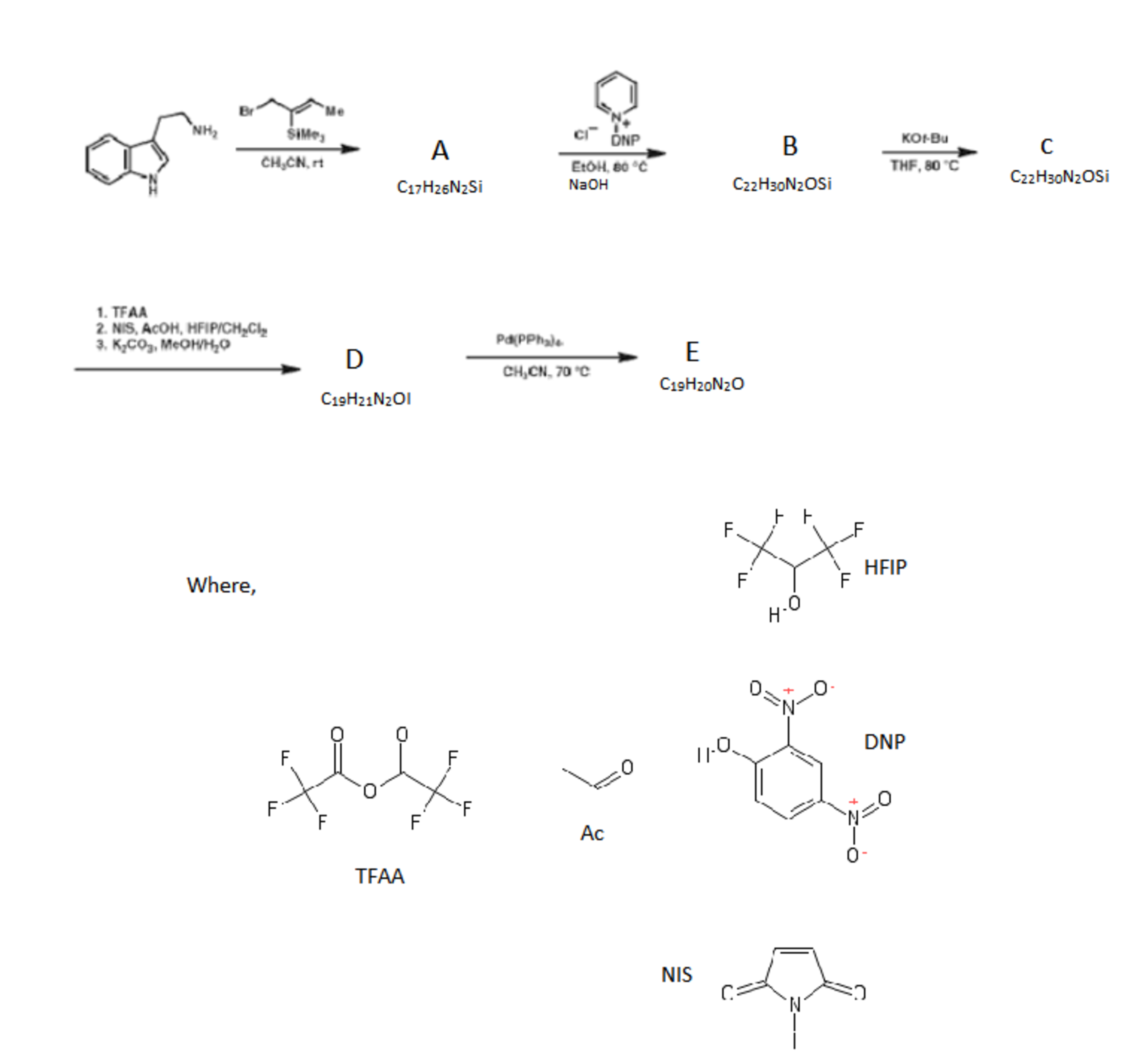
If denotes the number of rings in E and denotes the number of rings in Z
Calculate the value of
BONUS
- Deduce structures of W - Z and A - E
- Provide a mechanism for the conversion of Y to Z and B to C
All images belong to their respective owners.
The answer is 54.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
0 solutions
No explanations have been posted yet. Check back later!