Acidic Aromatic compounds.
Chemistry
Level
2
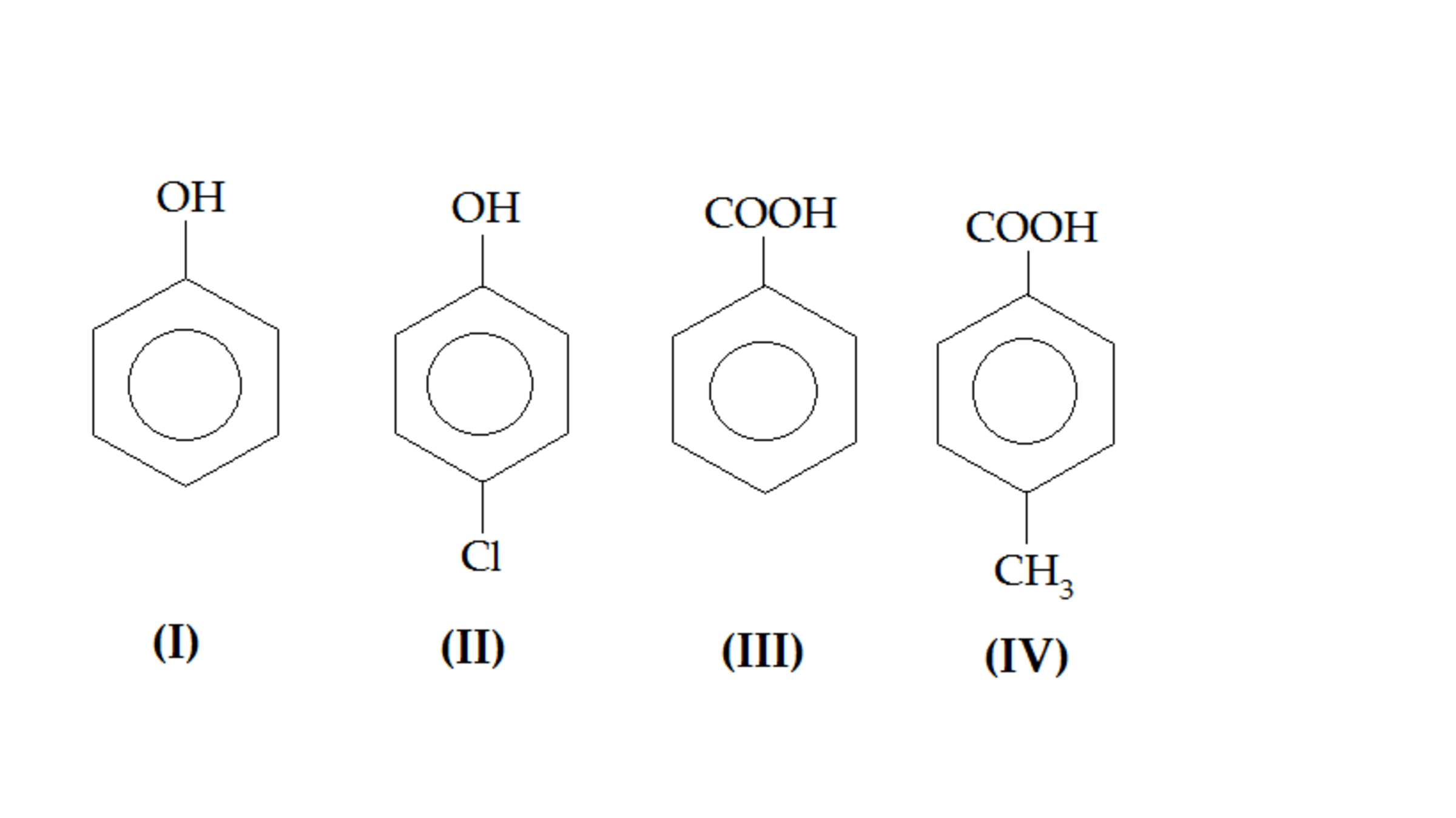
Which among the following has the 2nd highest acidic character?
Phenol
p-Toluic acid
Benzoic acid
Parachlorophenol
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Its quite obvious that Acid will have more acidic character than Phenol .
Out of that CH3 Being an Activating Group (Through Hyperconjugation or Inductive effect) will destabilise the conjugate base and hence decreasing Its Strength
But on Ortho substituted Carboxylic Acids this is not applicable there phenomenon Called Ortho Effect takes place