Addition reactions
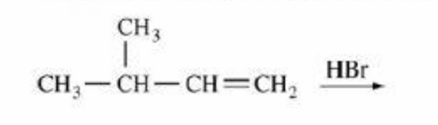 Which of the following is the major product of the reaction shown?
Which of the following is the major product of the reaction shown?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
3 solutions
Where on earth is option E ?
Log in to reply
Where is E seriously? how can you give me wrong? i clicked A?
There's just one little mistake, bro! It'll be 1,2-Methyl shift, not Hydride shift, because a methyl group is being shifted to make the more stable tertiary ( 3 ∘ ) carbocation.
Is this not Markonikov reaction?????
Log in to reply
Yeah, this is markovnikov addition but it is followed by 1,2-hydride shift to make the product stable.
it is but u have to first re arrange it to get a more stable product thus rearrangement takes place and three degree carbocation will be formed to gain more stability
"Simple", "of", "after", "since", "more", "stable". Would you please explain all other words. Thanks.
Log in to reply
Nothing much complicated here. It's just that this reaction will occur faster in Substitution Nucleophilic 1 process and in S N 1 process, the more substituents there is on the central carbon atom of the carbocation to be formed, the more stable the carbocation will be. Since tertiary carbocation is more stable than secondary carbocation, there will be Whitmore 1,2-shift to create the more stable ( 3 ∘ ) carbocation rather than the expected reaction route.
because it follows the markonikhofs rule any attacking nucleophile will attack to that carbon atom which contains less number of hydrogen atoms
except that by markovnikov, they would go in either of the atoms of the double bond which is broken in the reaction
As this might seem weird when you're used to witness Markonikov's reaction which would have given (CH3)2-CH-CH(Br)-CH3, it can be understood when you consider the formation of the carbocation. The carbocation formed by the breaking of the double bound on the second carbon is more stable when placed on a tertiary carbon by inductive effect. Therefore, molecular rearrangement happens after the addition of the hydrogen on the first carbon, so that the carbocation is placed on the tertiary carbon. Therefore, the Br- attacks the tertiary carbon, forming product A.
Simple re-arrangement of carbo-cation after protonation of alkene ! { By Hydride 1-2 shift ) since 3 degree carbo-cation is more stable ! :)