Adiabatic velocity!
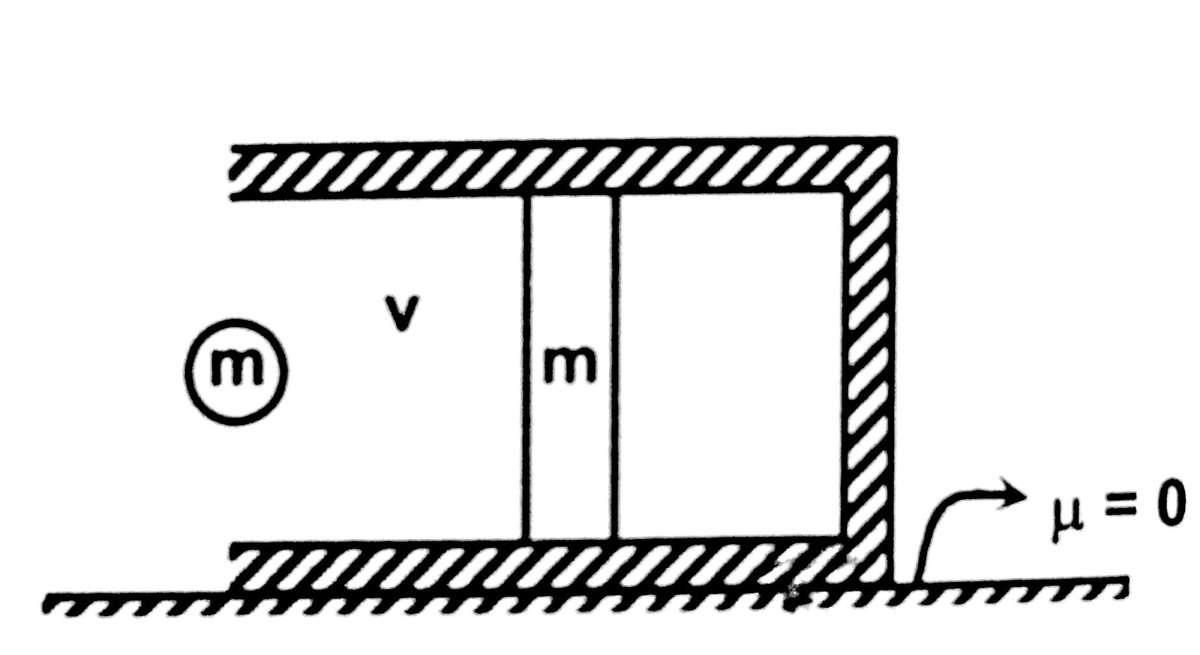
An adiabatic chamber has frictionless piston of mass . Mass of remaining chamber including gas is . moles of an ideal monoatomic gas is present inside the chamber at atmospheric temperature and pressure. Piston is at rest in equilibrium and confined to move along the length of the cylinder. A particle of mass m moving horizontally with speed , strikes elastically with the piston.
The temperature of the gas when the compression of the gas is maximum is of the form .
Find .
The answer is 20.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
0 solutions
No explanations have been posted yet. Check back later!