Alcoholic Coordination
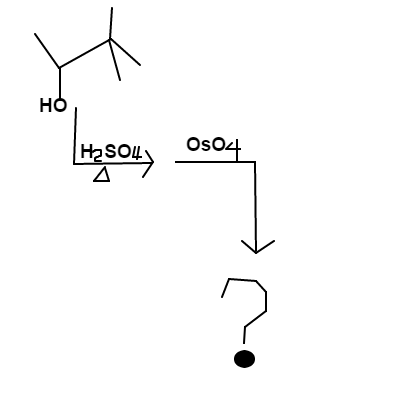 is mixed into a solution of heated
and the major product is mixed with
. The final product will contain one or more alcohol substituents.
Your answer should be the sum of the carbon positions (
) an alcohol is attached.
For example, if
,
, and
all have alcohol groups, then your answer would be
.
Use
IUPAC
numbering protocol when labeling the carbon positions in your answer.
is mixed into a solution of heated
and the major product is mixed with
. The final product will contain one or more alcohol substituents.
Your answer should be the sum of the carbon positions (
) an alcohol is attached.
For example, if
,
, and
all have alcohol groups, then your answer would be
.
Use
IUPAC
numbering protocol when labeling the carbon positions in your answer.
David's Organic Chemistry Set
David's Physical Chemistry Set
The answer is 5.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
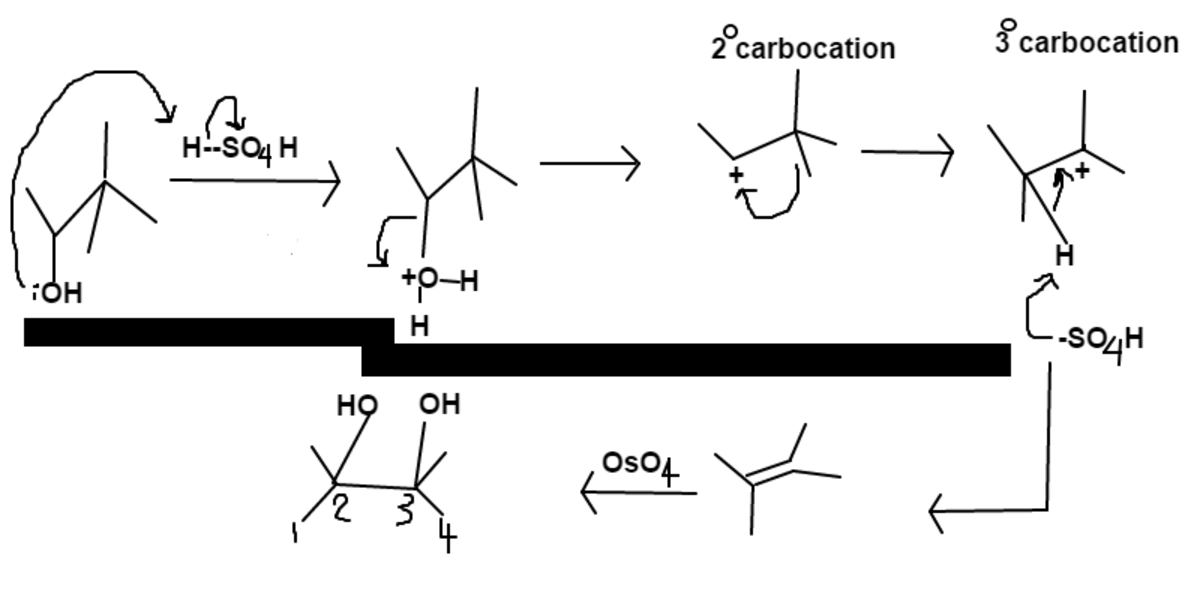 Notice the rearrangement to go from a
2
∘
carbocation to a more stable
3
∘
carbocation.
Once the alkene is formed, osmium tetroxide will add alcohols to
C
2
and
C
3
; thus
Answer
⇒
2
+
3
=
5
Notice the rearrangement to go from a
2
∘
carbocation to a more stable
3
∘
carbocation.
Once the alkene is formed, osmium tetroxide will add alcohols to
C
2
and
C
3
; thus
Answer
⇒
2
+
3
=
5