Aldehyde and ketone (just to learn 7)
Chemistry
Level
3
Canizaro -reaction mechanism is provided below which of the following is rate determining step?
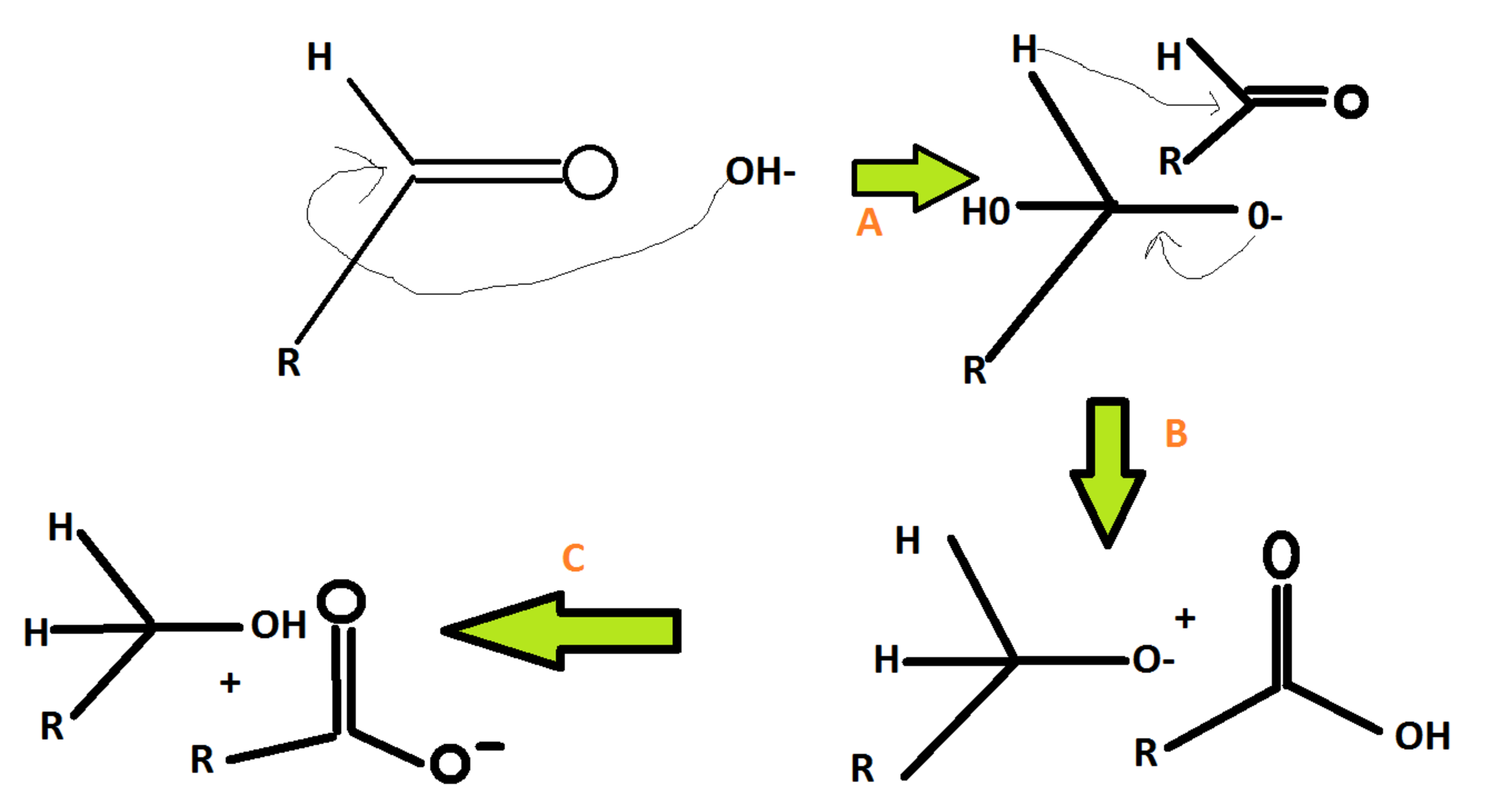
B
all are unidirectional fast steps
A
C
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
H- is a very poor leaving group . hence it leaves in rate determining step.
Intermolecular cannizaro are 3rd order reaxn's while intramolecular are 2nd order reaxn's