Alkene Crafting
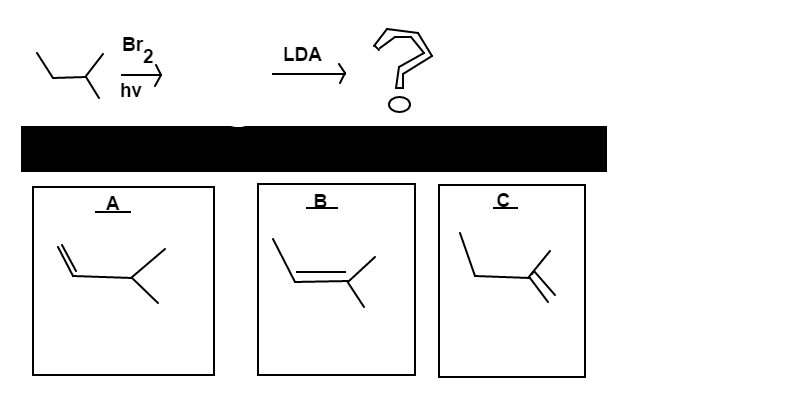 is mixed with
in light. This is followed up with an addition of
.
Determine which alkene;
,
, or
, will be the major product of this reaction scheme.
is mixed with
in light. This is followed up with an addition of
.
Determine which alkene;
,
, or
, will be the major product of this reaction scheme.
David's Organic Chemistry Set
David's Physical Chemistry Set
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
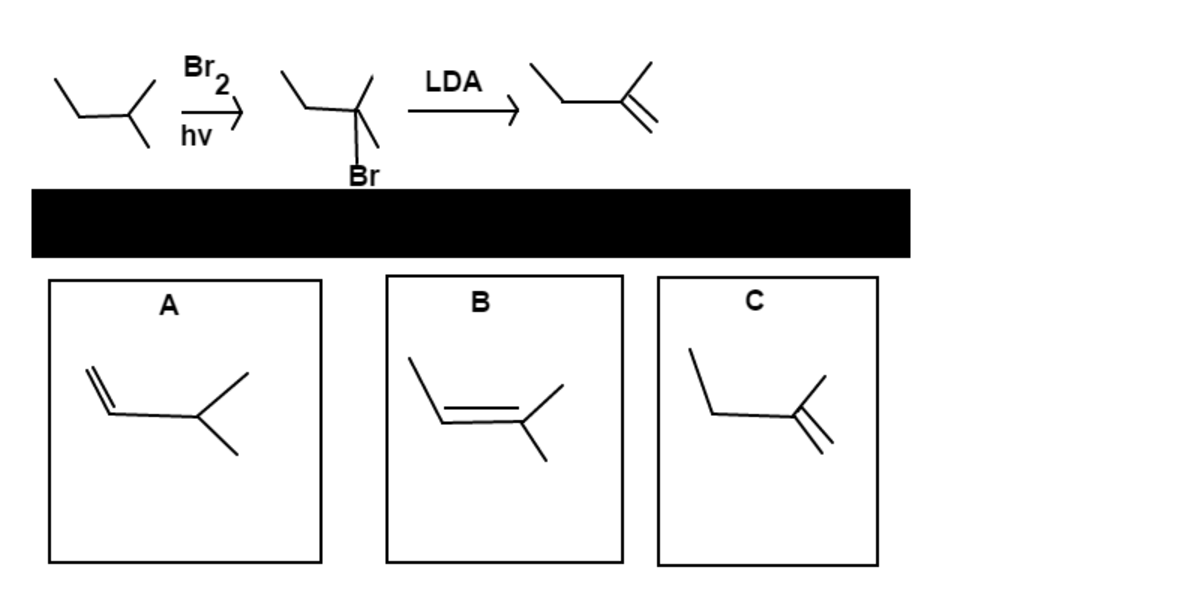 Firstly,
B
r
will be added to the most highly substituted carbon, which in this case is the
3
∘
carbon in
2
-
m
e
t
h
y
l
b
u
t
a
n
e
. Then
L
D
A
will remove the
B
r
, causing a double bond to form. The least substituted double bond in choice
C
is preferred over
B
because
L
D
A
is a bulky reagent.
A
n
s
w
e
r
→
C
Firstly,
B
r
will be added to the most highly substituted carbon, which in this case is the
3
∘
carbon in
2
-
m
e
t
h
y
l
b
u
t
a
n
e
. Then
L
D
A
will remove the
B
r
, causing a double bond to form. The least substituted double bond in choice
C
is preferred over
B
because
L
D
A
is a bulky reagent.
A
n
s
w
e
r
→
C