Amalgamations. Again
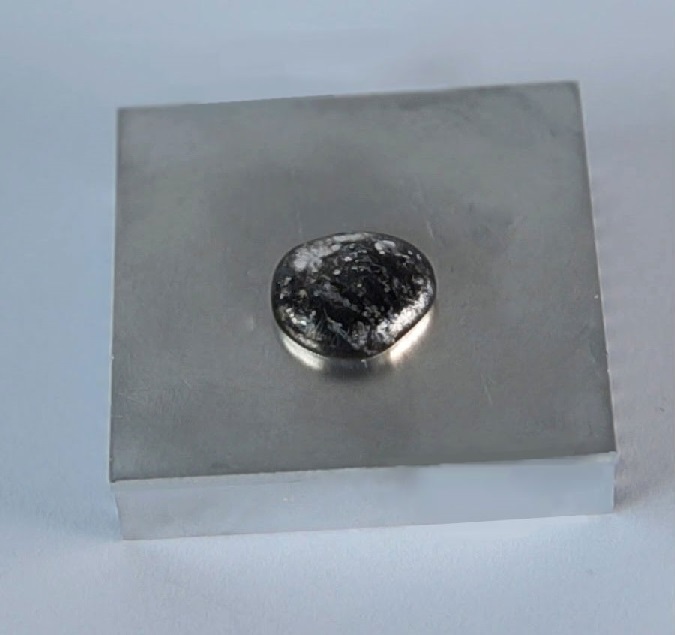
Aluminium can form an amalgam with mercury. However, when I put drops of liquid mercury on a solid aluminium, no reaction occurs.
Which is the most probable explanation of this situation?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Aluminium is usually covered with an oxide layer, preventing further reactions. Therefore, to cause a reaction between them, oxide layers should be removed by acids before pouring mercury on its surface.