An AC would surely help!
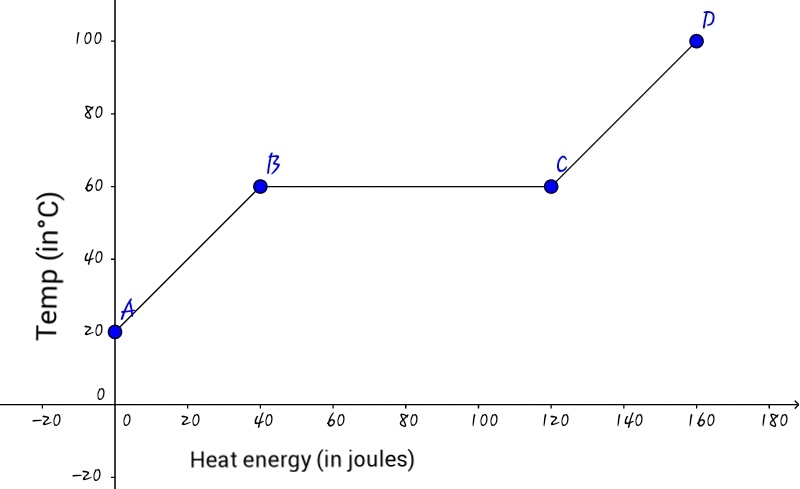
A solid initially at is heated. The graph shows variation in temperature with the amount of heat energy supplied to it. Now, if the specific heat capacity of the solid is calculate
(i) the mass of the solid and
(ii) the specific latent heat of fusion of the solid.
Submit your answer as the sum of (i) (in grams) and (ii) in
The answer is 160.5.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Relevant wiki: Heat Transfer
In part A B of the graph,
Heat required = 4 0 J .
Change in temperature = 4 0 ° C .
Using formula, H = m × c × θ R , 4 0 = m × 2 × 4 0 m = 2 1 m = 0 . 5 g
In part B C of the graph,
Heat required = ( 1 2 0 − 4 0 ) J = 8 0 J
We know that m = 0 . 5 g (above). Using formula, H = m × L ,
8 0 = 0 . 5 × L L = 0 . 5 8 0 L = 1 6 0 J / g
∴ The required answer is 0 . 5 + 1 6 0 = 1 6 0 . 5 .