Ancient aliens
NASA has captured an extraterrestrial space probe that has been in space for millions of years. The probe was operated by a kind of radionuclide battery, which on launch consisted of a pure block of plutonium-244. Now, the isotope ratio between thorium-232 and plutonium-244 is 1:1 due to the radioactive decay ( N Pu = N Th ) .
How long was the probe in space? (Specify the time in millions of years and round it to the nearest integer.)
Details and Assumptions:
- Plutonium-244 decays in a two-step reaction into thorium-232: 2 4 4 Pu ⟶ 2 3 6 U ⟶ 2 3 2 Th .
- The half-lives of plutonium-244 and uranium-236 are 80 and 23 million years, respectively.
- Thorium-232 is stable (half-life is longer than 10 billion years).
The answer is 99.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
2 solutions
A single decay can be descripted by an exponential function
N
(
t
)
=
N
0
exp
(
−
λ
t
)
with the initial number of atoms
N
0
and the decay constant
λ
.
The decay constant can be calculated from the half-life time:
2
N
0
=
N
(
t
1
/
2
)
=
N
0
exp
(
−
λ
t
1
/
2
)
⇒
λ
=
t
1
/
2
ln
2
For a two-step decay we have a system of differential equations
N
˙
Pu
N
˙
U
N
˙
Th
=
−
λ
Pu
N
Pu
=
λ
Pu
N
Pu
−
λ
U
N
U
=
λ
U
N
U
for the number of atoms
N
X
for
X
=
Pu
,
U
,
Th
.
Initial conditions for
t
=
0
are
N
Pu
=
N
0
,
N
U
=
N
Th
=
0
.
The solution for
N
Pu
is a simple exponential decay:
N
Pu
(
t
)
=
N
0
exp
(
−
λ
Pu
t
)
For uranium a general solution of the homogeneous equation
N
˙
U
+
λ
U
N
U
=
0
⇒
N
U
∝
exp
(
−
λ
U
t
)
and a special solution of the inhomogeneous equation
N
˙
U
+
λ
U
N
U
=
λ
Pu
N
Pu
⇒
N
U
∝
exp
(
−
λ
Pu
t
)
can be found, so that the general solution is linear combination of both:
⇒
⇒
N
U
(
t
)
N
˙
U
λ
Pu
N
Pu
−
λ
U
N
U
A
N
U
(
0
)
B
=
A
exp
(
−
λ
Pu
t
)
+
B
exp
(
−
λ
U
t
)
=
−
λ
Pu
A
exp
(
−
λ
Pu
t
)
−
λ
U
B
exp
(
−
λ
U
t
)
=
(
λ
Pu
N
0
−
λ
U
A
)
exp
(
−
λ
Pu
t
)
−
λ
U
B
exp
(
−
λ
U
t
)
=
λ
U
−
λ
Pu
λ
Pu
N
0
=
A
+
B
=
!
0
=
−
A
=
−
λ
U
−
λ
Pu
λ
Pu
N
0
The number of thorium atoms can be found by integration:
N
Th
(
t
)
=
λ
U
∫
0
t
N
U
(
t
)
d
t
=
−
λ
U
[
λ
Pu
A
exp
(
−
λ
Pu
t
)
+
λ
U
B
exp
(
−
λ
U
t
)
]
0
t
=
λ
U
−
λ
Pu
λ
U
N
0
[
1
−
exp
(
−
λ
Pu
t
)
]
−
λ
U
−
λ
Pu
λ
Pu
N
0
[
1
−
exp
(
−
λ
U
t
)
]
=
N
0
−
N
Pu
(
t
)
−
N
U
(
t
)
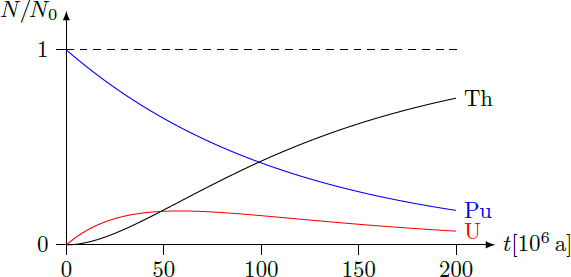
A plot of the functions provides the graphical solution
N
Pu
(
t
)
=
N
Th
(
t
)
for the age
t
≈
9
9
⋅
1
0
6
a
(freepascal)
program desintegration;
uses math;
var
cpr,ctr,ctr0,dt,a,b,c,alfa,beta,gamma:real; n:longint;
begin
alfa:=ln(2)/80000000;
beta:=ln(2)/23000000;
dt:=1000; { step of time: 1000 years }
a:=exp(-(dt*alfa));
b:=exp(-(dt*beta));
c:=dt*alfa;
ctr0:=c*a/(1-a);
gamma:=0;
n:=0;
while ctr<=cpr do
begin
cpr:=(1-c*(1- exp((n+1)*ln(a)) )/(1-a) ); {plutonium quantity after n thousands of years }
gamma:=exp(n*ln(a))+b*(gamma);
ctr:=ctr0*((1-exp((n+1)*ln(b)))-(1-b)*gamma); {Thorium quantity after n thousands of years }
n:=n+1
end;
n:=round(n/1000);
writeln(n); { number of thousands of years }
end.
Result: n=99
great problem, sir !
The decay can be described as a matrix differential equation d t d ⎣ ⎡ Pu U Th ⎦ ⎤ = ⎣ ⎡ − α α 0 0 − β β 0 0 0 ⎦ ⎤ ⎣ ⎡ Pu U Th ⎦ ⎤ . Here, α = halflife of Pu ln 2 ; β = halflife of U ln 2 . In general, to solve a linear matrix differential equation of the form d x / d t = M x , we wish to find a basis for the vector space in which M is diagonal. Then we simply apply d t d x = ⎣ ⎡ λ 1 ⋱ λ n ⎦ ⎤ x ; x ( 0 ) = ⎣ ⎢ ⎡ a 1 ⋮ a n ⎦ ⎥ ⎤ ; ⟹ x ( t ) = ⎣ ⎢ ⎡ a 1 e λ 1 t ⋮ a n e λ n t ⎦ ⎥ ⎤ . So let's get to work. To find the eigenvalues, we solve 0 = det ( M − λ I ) = ∣ ∣ ∣ ∣ ∣ ∣ − α − λ α 0 0 − β − λ β 0 0 − λ ∣ ∣ ∣ ∣ ∣ ∣ = − ( α + λ ) ( β + λ ) λ . Thus the eigenvalues are λ 1 = − α ; λ 2 = − β ; λ 3 = 0 . The corresponding eigenvectors are e 1 = ⎣ ⎡ β − α α − β ⎦ ⎤ ; e 2 = ⎣ ⎡ 0 1 − 1 ⎦ ⎤ ; e 3 = ⎣ ⎡ 0 0 1 ⎦ ⎤ . The original amounts may be written as ⎣ ⎡ Pu 0 U 0 Th 0 ⎦ ⎤ = ⎣ ⎡ 1 0 0 ⎦ ⎤ = a 1 ⎣ ⎡ β − α α − β ⎦ ⎤ + a 2 ⎣ ⎡ 0 1 − 1 ⎦ ⎤ + a 3 ⎣ ⎡ 0 0 1 ⎦ ⎤ ; it is not difficult to see that a 1 = β − α 1 , a 2 = β − α − α , a 3 = 1 . Thus, translated to the eigenvalue bases, the process is now reduced to the diagonal form d t d x = ⎣ ⎡ − α − β 0 ⎦ ⎤ x ; x ( 0 ) = β − α 1 ⎣ ⎡ 1 − α β − α ⎦ ⎤ with solution x ( t ) = β − α 1 ⎣ ⎡ e − α t − α e − β t β − α ⎦ ⎤ . Translated back to the original basis, we now have an explicit formula for the amounts of each substance : ⎣ ⎡ Pu U Th ⎦ ⎤ ( t ) = β − α 1 ⎣ ⎡ ( β − α ) e − α t α ( e − α t − e − β t ) β ( 1 − e − α t ) − α ( 1 − e − β t ) ⎦ ⎤ .
To answer the question, we equate the amounts of plutonium and thorium: ( β − α ) e − α t = β ( 1 − e − α t ) − α ( 1 − e − β t ) ; 2 . 4 0 3 5 ⋅ 2 − t / 8 0 − 0 . 4 0 3 5 ⋅ 2 − t / 2 3 = 1 . There is no simple algebraic solution; a numerical solution yields t ≈ 9 9 million years.]