Aromatics, Acylation, And Bromination
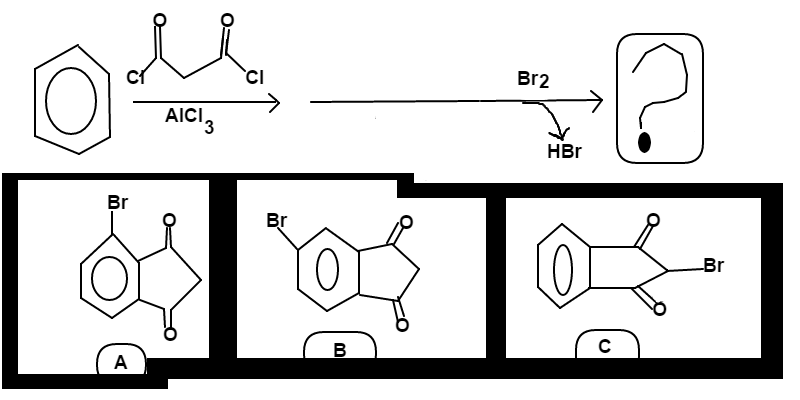 is reacted with
in the presence of
, and this is followed by an addition of
to the reaction.
is reacted with
in the presence of
, and this is followed by an addition of
to the reaction.
Pick the major product from choices , , or .
David's Organic Chemistry Set
David's Physical Chemistry Set
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
First, Friedel Craft Acylation occurs to form a bicyclic compound
B r 2 is incapable, by itself, of brominating the aromatic ring; however, the carbon alpha to both ketones has highly acidic hydrogens. B r 2 will react with one of these acidic hydrogens, forming H B r and brominating the carbon with the second B r
A n s w e r = C