Atom
Chemistry
Level
1
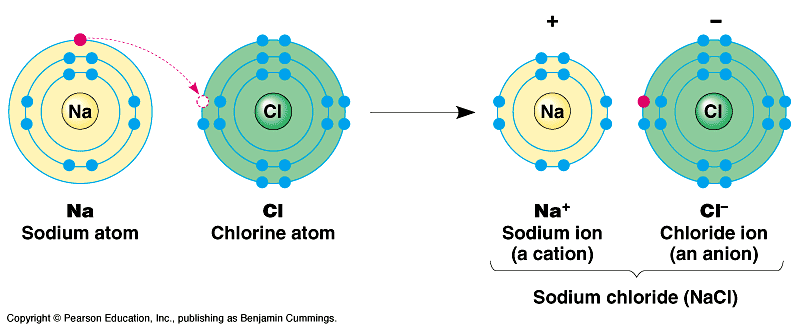
The atomic number of X is 11.
The atomic number of Y is 17. The neutron number of Y is 18.
Is it possible for X atoms to create a stable structure with Y atoms?
Yes
No
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
X and Y atom's Electron shells Is able to create stricture. because of ionic bond **The atomic number of X is 11 it means that it is SODIUM(Na) and Y atom's atomic number is 17 and neutron number is 18. It means it is chlorine(Cl). in sodium chloride a sodium atom loses its one valence electron to form sodium ion and chlorine atom accepts one electron to form chloride ion to form chloride ion. Due to opposite charges, both ions attract each other and form sodium chloride; NaCl.