Back to the basics of coordination chemistry
If you already know about Sidgwick's EAN rule, scroll down to skip to the main problem.
The Sidgwick's rule of effective atomic number (EAN rule) states that in a number of metal complexes, the metal atoms tend to surround themselves with ligands in a way that their effective atomic number is numerically equal to the noble gas element found in the same period. Commonly most complexes are formed by the d-block elements, therefore the most frequently referred noble gases are Krypton(36), Xenon(54) and Radon(86).
The formula to compute the effective atomic number of the metal atom in a complex is given by:
However, this rule was later ruled out when Crystal Field Theory was published years later which clearly stated the reasons for the stability of complexes.
The EAN rule is still, however, an interesting concept and is frequently a topic for problems in coordination chemistry.
Problem:
Assuming that EAN rule holds and the given metal (where metal atom belongs to the fifth period of the periodic table) complexes are stable, find the metal atoms in each complex and their oxidation states.
Let the atomic numbers of the metal atoms in each respective complexes and are and and their oxidation states are and respectively. Calculate .
Details:
-
The ligand is the tricyclohexylphosphine ligand.
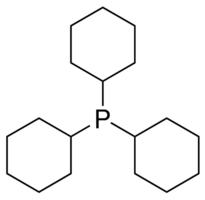
-
In figure C, the biphenyl group present in the figure connected by a bond joining metal atom to only one carbon atom of each benzene ring has only 2 participating electrons per bond .
The answer is 163.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
0 solutions
No explanations have been posted yet. Check back later!