Balance it out.
Consider the following steady-state open system, involving flow of three substances
and
:
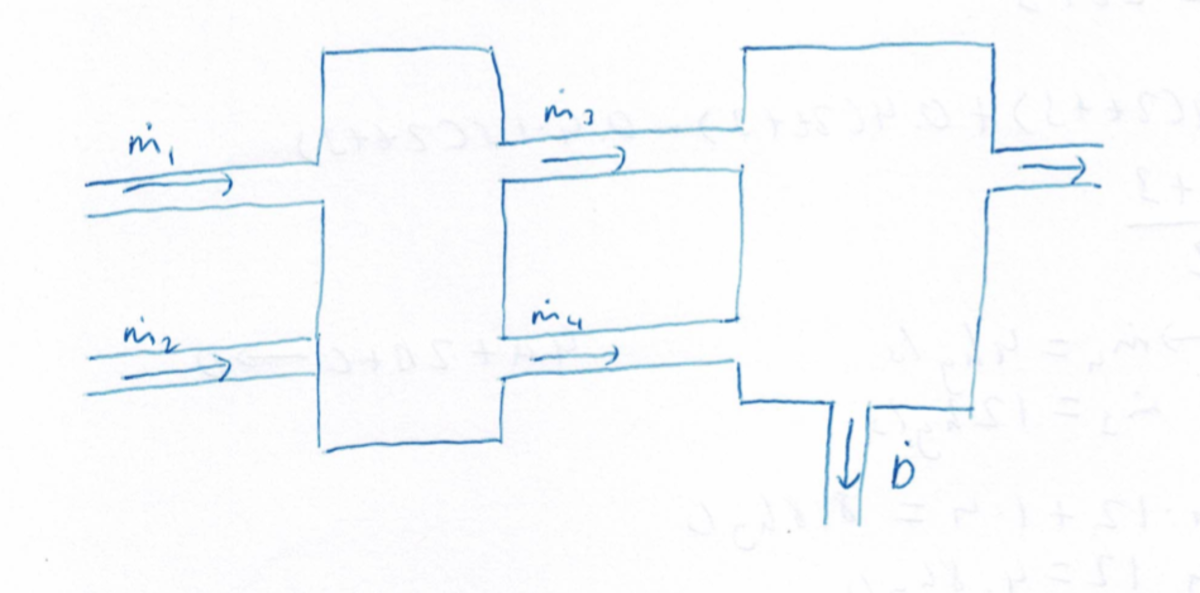
Two streams and , of mass flow rates and respectively enter the first chamber. has a variable mass flow rate of where is time in seconds. Two other streams and , of mass flow rates and respectively exit it. These two streams which exit the first chamber also enter a second chamber, a reaction chamber. In this chamber, and react to form another substance through the reaction equation . All excess reactants are ejected through another stream going out of the reaction chamber. Calculate the mass flow rate of after seconds, in .
Additional information :
- Stream is and the rest .
- Stream is and the rest .
- Stream is and also outputs equivalent amounts of and .
- Stream comprises of only .
- Assume that and all have the same molar mass.
The answer is 924.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
m1 = m2 & m3 = 3 × m4
There should be 55% of A, 30% of B and 15% of C in the reaction chamber. A would be wholly utilized and left 3.75% (B+C mixture) as excess reactants.
m1(2.5) = 2 × 2.5 + 3 = 8 kg/s
Total mass of inputs
= m1 + m2
= 8 + 8
= 16 kg/s
Answer
= (100% – 3.75%) × 16 kg/s
= [ 15.4 kg/s ] × 60s
= 924 kg/minute