Balancing chemical equations: TNT
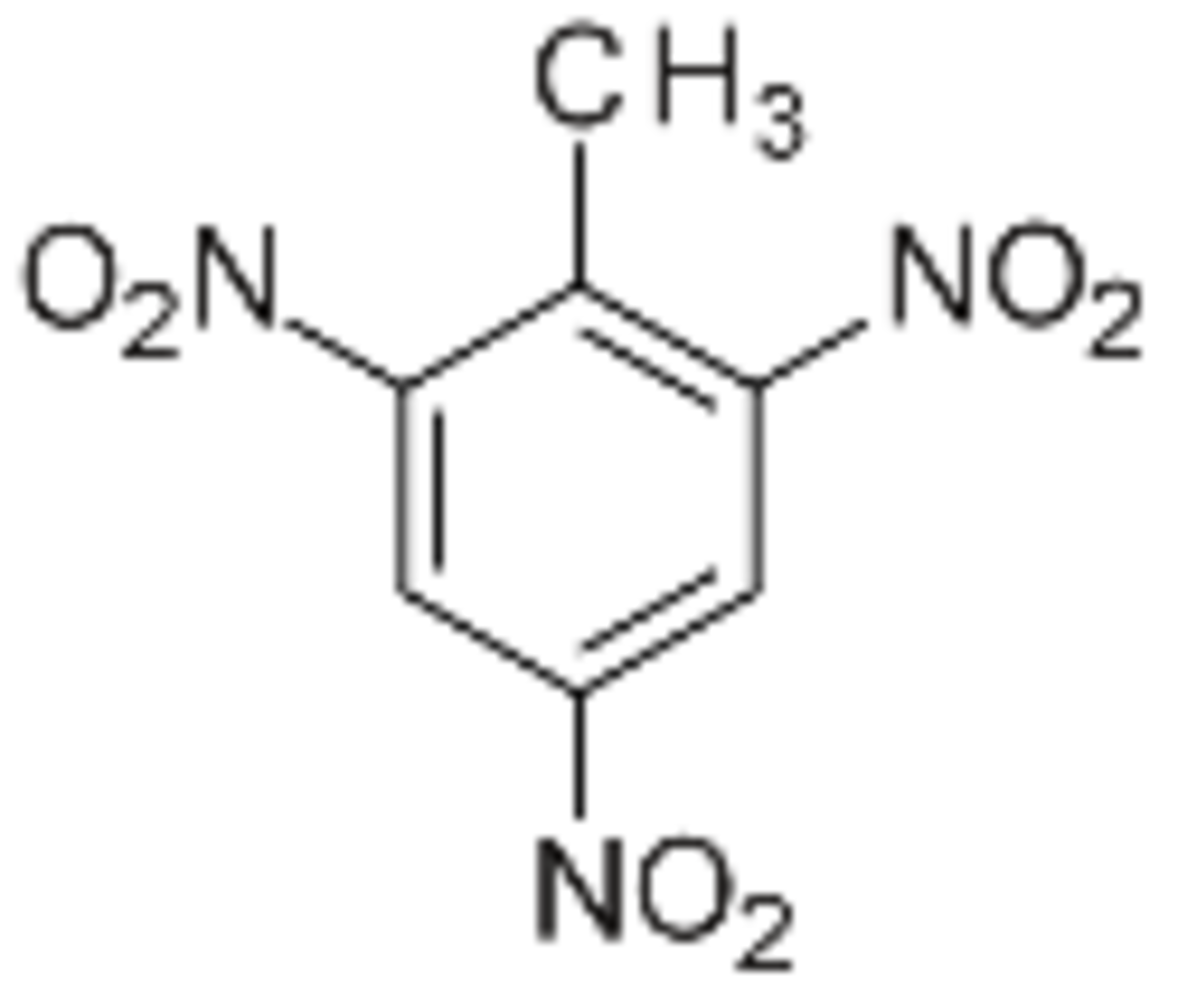 TNT: C7H5N3O6
TNT: C7H5N3O6
Trinitrotoluene (TNT) is a common material used in explosives. Upon detonation, the decomposition of TNT can proceed in the following 2 reactions:
-
1 mole (mol.) of TNT producing nitrogen, water, carbon monoxide and carbon.
-
1 mol. of TNT producing nitrogen, hydrogen, carbon monoxide and carbon.
Given complete reaction and that all products are returned to room temperature and pressure (25 deg C at 1 atm), How many more cubic decimetres ( ) of gaseous products are produced per mole of TNT in reaction (2) compared to per mole of TNT in reaction (1)?
More in this series of problems for balancing chemical equations: Alcohol redox | TNT | CuSCN-KIO3 system
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
0 solutions
No explanations have been posted yet. Check back later!