Ball Packing Wars
At the ball-making factory, two workmen are packing identical spherical balls inside identical cube boxes.

Workman Alex is using the hexagonal packing method. Workman Bill is using the face-centered cubic packing method. The following describes the two methods
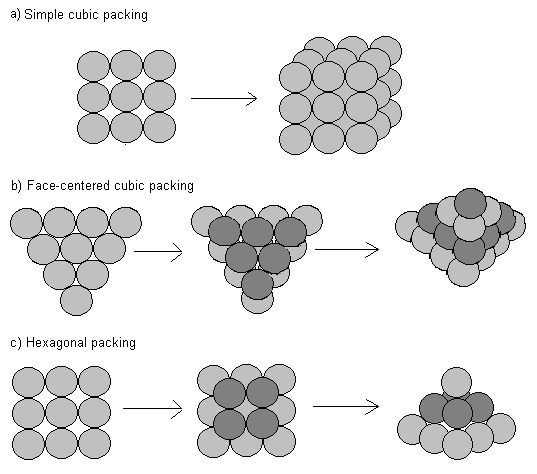
Neither is using the simple cubic packing, because they both know that it's an inefficient way to pack balls in boxes. Both are close-packing the balls, meaning that the balls are tightly packed, and any gaps between the stack of balls and the inside walls of the cube boxes are filled with packing material. They get into an argument about who's packing more efficiently.
Alex says, "We know that both methods will ultimately have the exact same packing density, which is or about %, but that's true only as a limit. Because this box has right angles, it's better that I start with balls packed in a square array at the bottom, and work my way up. It is so obvious."
Bill says, "Maybe obvious to you, but I find that by using the hexagonal array, I have more opportunities to pack more balls around the perimeter. That does depend on the exact size of the box, though. I just prefer to pack this way, it's more flexible."
They bicker and they quibble, but soon after a while, they both finish packing their identical cube boxes with the maximum possible number of balls, using each of their preferred packing methods. To their surprise, they've packed exactly the same number of balls!
Given that the number of balls is more than 3 dozen, what is the smallest number of balls each could have packed in their identical cube boxes?
It's assumed that both packers start with a layer of a maximum number of balls at the bottom of the box, using their preferred packing methods, and work up a layer at a time.
Reference Note:
If the balls have a radius of , then
For hexagonal packing
Spacing between levels
Spacing between rows
For face-centered cubic packing
Spacing between levels
Spacing between rows
The answer is 1500.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
0 solutions
No explanations have been posted yet. Check back later!