Bicyclo[3.3.0] Octane
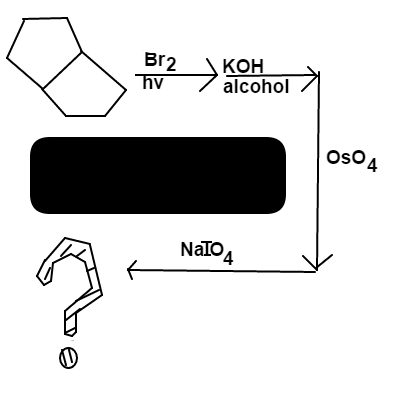 is reacted in the following order:
is reacted in the following order:
-
in
-
in
-
( )
-
( )
What will be the for the ?
David's Organic Chemistry Set
David's Physical Chemistry Set
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
B r first adds to one of the preferred 3 ∘ carbons
K O H will remove the B r to form a double bond. The most substituted double bond between the two 3 ∘ carbons is preferred.
O s O 4 forms a diol among the double bonded carbons
Lastly, I O 4 breaks the bond between the diol and forms ketones with the alcohols
The final product is 1 , 5 c y c l o o c t a d i o n e A n s w e r = 1 D . U . ( r i n g ) + 2 D . U . ( c a r b o n y l ) = 3 D . U . D . U . = D e g r e e s o f U n s a t u r a t i o n