Bohr's orbit
An electron with charge revolves around a nucleus of an element with atomic number Find the value of when of energy is needed to excite the electron from the second Bohr's orbit to the third.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Firstly, let's convert 4 7 . 2 e V into joules. This can be done so using the formula, title
title
Where V is the voltage, W is the work done in joules and q is the charge of the electron, which is about 1 . 6 0 2 × 1 0 − 1 9 C .
Then we may have to formulate Bohr's equation for energy by combining together the Rydberg formula and Planck's law.
Which leads us to this result,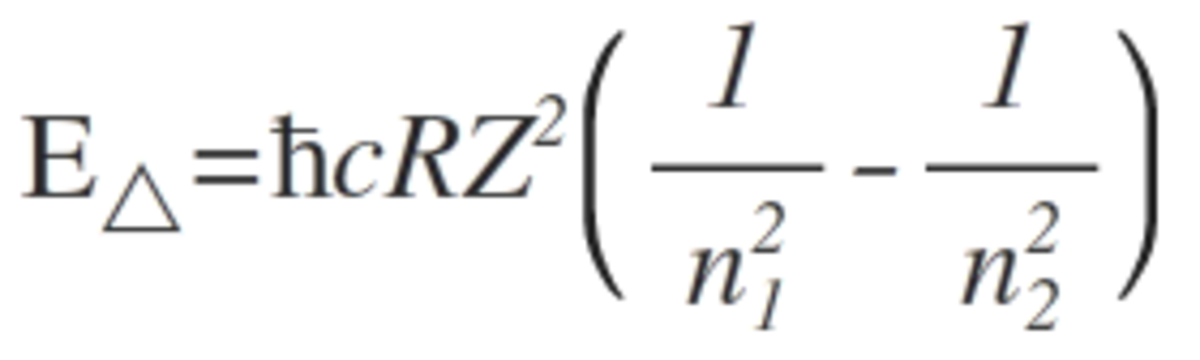
Where E △ is the energy change, Z is the atomic number of the element, R is the Rydberg constant, h is the planck's constant, c is the speed of light and n will be the principal quantum numbers of the orbitals. Substituting the values yield a value close to 5 which is the answer.