Breaking Bad
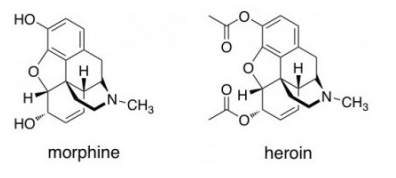 You recently turned 50 and you have just been diagnosed with terminal lung cancer. You decide to try and make as much money as possible before you die so that your family can live comfortably. You turn to synthesizing heroin as you heard there is a lot of money in the business and that methylamine would be too difficult to access to synthesize methamphetamine. Little did you know morphine is also a difficult reactant to get your hands on. The chemical structure of morphine is given in the attached photograph. What other chemical would you mix with morphine if you wanted to create heroin as a product?
You recently turned 50 and you have just been diagnosed with terminal lung cancer. You decide to try and make as much money as possible before you die so that your family can live comfortably. You turn to synthesizing heroin as you heard there is a lot of money in the business and that methylamine would be too difficult to access to synthesize methamphetamine. Little did you know morphine is also a difficult reactant to get your hands on. The chemical structure of morphine is given in the attached photograph. What other chemical would you mix with morphine if you wanted to create heroin as a product?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.

This is a simple case of Fischer Esterification . When you mix an alcohol with a carboxylic acid, there is loss of water, to form an ester.
In this case, I have marked the two alcoholic positions. When Ethanoic Acid ( C H 3 C O O H ) is added to the Morphine, the following reaction takes place :
Note: The above reaction occurs at both the alcoholic positions, thus giving us Heroine as the product.