Bromine Hopping
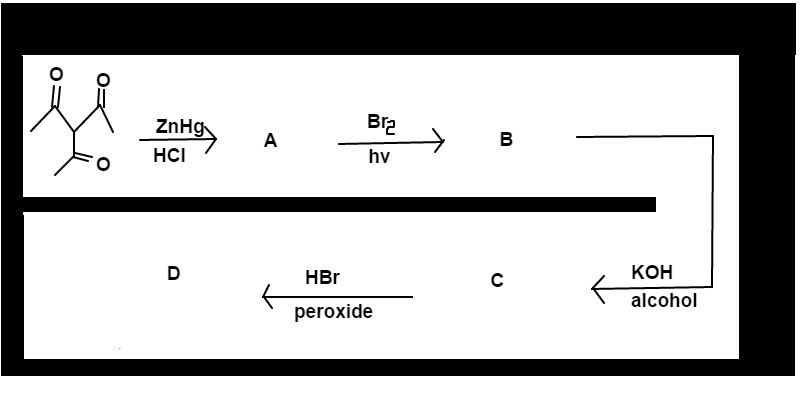 Starting with
3-acetyl-2,4-pentadione
, precede through the reactions shown above.
and
will both be products that have the standard name:
-bromo-3-ethyl pentane
. You must determine which carbon (
) positions the Bromine will be in products
and
. Find the difference of those positions going from product
to
.
Starting with
3-acetyl-2,4-pentadione
, precede through the reactions shown above.
and
will both be products that have the standard name:
-bromo-3-ethyl pentane
. You must determine which carbon (
) positions the Bromine will be in products
and
. Find the difference of those positions going from product
to
.
For example, if the Bromine is on of product and of product then your answer will be .
Use IUPAC numbering protocol when answering this question.
David's Organic Chemistry Set
David's Physical Chemistry Set
The answer is 1.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Step 2 : Addition of Bromine onto most highly substituted carbon : c 3
Step 3 : Removal of Bromine molecule to form a double bond
Step 4: Anti Markovnikov addition of Bromine onto least substituted carbon in double bond : c 2