Butadiene
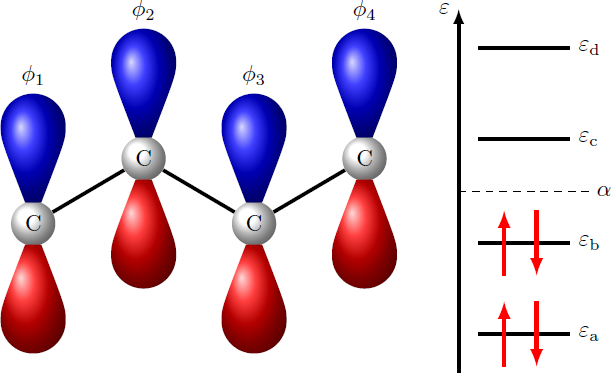
Butadiene (H 2 C = CH − CH = CH 2 ) is a four-carbon molecule arranged in a chain. In this molecule, there are π -bonds that result from the interactions of the electrons in the 2 p z -orbitals of carbon. The wave function ψ of the π -electrons can be approximated as a linear combination of the atomic orbitals: ψ ≈ i = 1 ∑ 4 λ i ϕ i where ϕ i denotes the 2 p z -orbital at the i -th carbon atom ( i = 1 , 2 , 3 , 4 ). The λ i ∈ R are the coefficients, which form a vector λ = ( λ 1 , λ 2 , λ 3 , λ 4 ) . The π -states result as a solution of the matrix equation = H ⎝ ⎜ ⎜ ⎛ α β 0 0 β α β 0 0 β α β 0 0 β α ⎠ ⎟ ⎟ ⎞ ⋅ ⎝ ⎜ ⎜ ⎛ λ 1 λ 2 λ 3 λ 4 ⎠ ⎟ ⎟ ⎞ = ε ⋅ ⎝ ⎜ ⎜ ⎛ λ 1 λ 2 λ 3 λ 4 ⎠ ⎟ ⎟ ⎞ with energy parameters α , β ∈ R . The quantity ε denotes an energy eigenvalue of the Hamiltonian matrix H . There are a total of four energy eigenvalues ε a < ε b < ε c < ε d as shown above in the energy diagram. In the ground state, the π -electrons have the total energy E = 2 ( ε a + ε b ) = 4 α − ξ ⋅ ∣ β ∣ What is the numerical value of ξ ?
Hint: The eigenvalues ε of H results as solutions of the characteristic polynomial det ( H − ε E ) = 0 , where E denotes the unit matrix ,
The answer is 4.47213595499958.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
This problem is an example of the application of the Hückel method .
We look for solutions of the characteristic polynomial det ( H − ε E ) = ∣ ∣ ∣ ∣ ∣ ∣ ∣ ∣ α − ε β 0 0 β α − ε β 0 0 β α − ε β 0 0 β α − ε ∣ ∣ ∣ ∣ ∣ ∣ ∣ ∣ = β 4 ∣ ∣ ∣ ∣ ∣ ∣ ∣ ∣ x 1 0 0 1 x 1 0 0 1 x 1 0 0 1 x ∣ ∣ ∣ ∣ ∣ ∣ ∣ ∣ = 0 , x = β α − ε The determinant can be calculated using the Laplace formula ∣ ∣ ∣ ∣ ∣ ∣ ∣ ∣ x 1 0 0 1 x 1 0 0 1 x 1 0 0 1 x ∣ ∣ ∣ ∣ ∣ ∣ ∣ ∣ ⇒ x 2 ⇒ x a,b,c,d = x ⋅ ∣ ∣ ∣ ∣ ∣ ∣ x 1 0 1 x 1 0 1 x ∣ ∣ ∣ ∣ ∣ ∣ − 1 ⋅ ∣ ∣ ∣ ∣ ∣ ∣ 1 1 0 0 x 1 0 1 x ∣ ∣ ∣ ∣ ∣ ∣ = x ⋅ ( x ⋅ ∣ ∣ ∣ ∣ x 1 1 x ∣ ∣ ∣ ∣ − 1 ⋅ ∣ ∣ ∣ ∣ 1 1 0 x ∣ ∣ ∣ ∣ ) − 1 ⋅ ( 1 ⋅ ∣ ∣ ∣ ∣ x 1 1 x ∣ ∣ ∣ ∣ − 0 ⋅ ∣ ∣ ∣ ∣ 1 0 1 x ∣ ∣ ∣ ∣ ) = x ⋅ ( x ⋅ ( x 2 − 1 ) − 1 ⋅ ( x − 0 ) ) − 1 ⋅ ( 1 ⋅ ( x 2 − 1 ) − 0 ) = x 4 − 3 x 2 + 1 = ! 0 = 2 3 ± 4 9 − 1 = 2 3 ± 5 = 4 ( 1 ± 5 ) 2 = 2 1 + 5 , 2 5 − 1 , − 2 5 − 1 , − 2 1 + 5 The results correspond to the golden ratio ϕ = 2 1 + 5 and his "little brother" ϕ = 2 1 − 5 . The ground state energy results to E = 2 ε a + 2 ε b = 4 α − 2 β ( x a + x b ) = 4 α − 2 5 β so that ξ = 2 5 .