Can we boil water in a paper cup?
Classical Mechanics
Level
1
When we place a paper cup on an open flame, it immediately catches fire and burns.
Is it possible to boil water in a paper cup on an open flame?
Yes
No
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
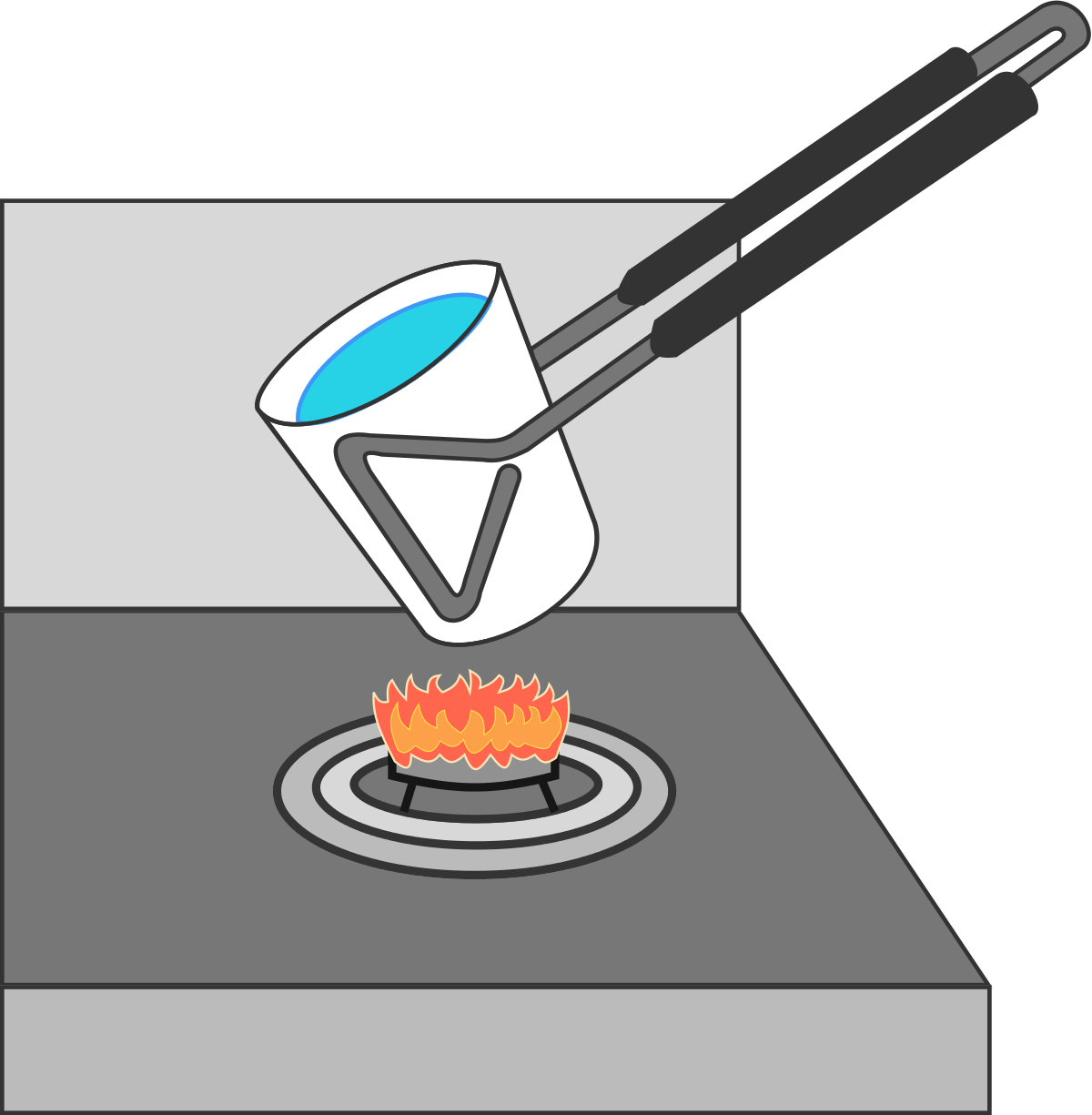
When an empty paper cup is put on a burner, it receives a lot of energy from it but can not pass the same to the surroundings as the air is a bad conductor of heat. Due to this, the energy of the paper cup increases rapidly and so thus its temperature. The paper cup catches fire as soon as its temperature is increased beyond its ignition temperature which is around 2 3 3 ∘ C .
Now, when we fill the cup with water and put it on the burner, the paper being very thin, conducts most of the energy directly to the water. Water is a good conductor of heat and thus it absorbs the heat rapidly and warms up.
Water boils at 1 0 0 ∘ C which is much smaller than the ignition temperature of the paper. Also, during the phase change from the liquid state to vapors, the temperature of the water remains fixed at its boiling point. Water boils and does not allow the temperature to rise beyond 1 0 0 ∘ C and hence the paper cup does not catch fire.