Can you estimate?
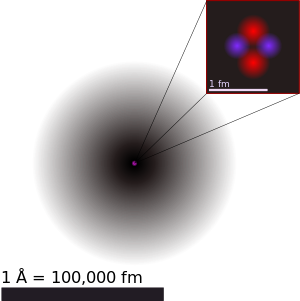 The above is the diagram of a helium atom, showing the electron probability density as shades of gray. The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons. By the way, Lithium (
Li), Beryllium (
Be), Boron (
B) and Carbon (
C) have the same number of electron shells but different atomic radii. Which one has the largest atomic radius?
The above is the diagram of a helium atom, showing the electron probability density as shades of gray. The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons. By the way, Lithium (
Li), Beryllium (
Be), Boron (
B) and Carbon (
C) have the same number of electron shells but different atomic radii. Which one has the largest atomic radius?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
atomic number and atomic radius is inversely proportional .in this case lithium has less atomic number so it has larger radius