Candle water problem
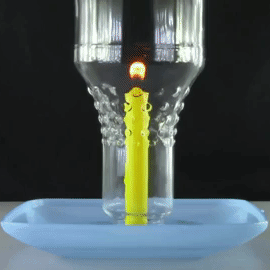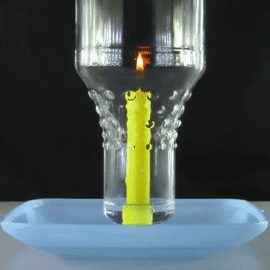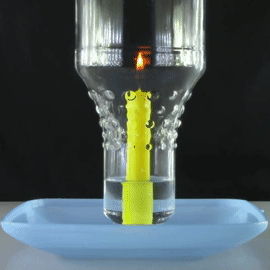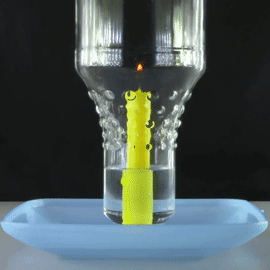
A bottle is placed over a lit candle sitting in a bowl of water.
The amount of oxygen the candle can use is more than the C O X 2 it produces, so water is sucked up into the bottle from the bowl, making the water level inside the bottle slowly rise.
What will happen to this water level when there is no more oxygen left in the bottle and the candle is extinguished?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
12 solutions
The pressure in the bottle remains constant. At constant pressure, the ratio V / T remains constant. The decrease in temperature T results in a decrease in volume V .
The pressure would increase if the water were not able to move.
Log in to reply
If the pressure remains the same, then which force will balance the weight of the water that is risen in the bottle?
Log in to reply
Right. One way to think about this precisely is by hydrostatic pressures. The pressure P at the boundary between the air and the water in the bottle is related to the ambient [atmospheric] pressure P 0 by P − P 0 = γ h where γ is the specific weight of water and h is the height of the water in the bottle above the surface of the water outside the bottle. Since P 0 and γ are effectively constant, Δ P ∝ Δ h
The pressure falls instantaneously, which allows the water to rise further, reducing the volume and equalising the pressure with the outside pressure.
It's not just about temperature. Carbon dioxide is far more soluble in water than is the oxygen it replaces, and on dissolving in the water reduces the amount of gas present and decreases the volume. Also, some of the combustion products of the flame are water vapour, which will condense on cooling and increase this effect.
Log in to reply
Yes! I wasn't going to mention this, but you're absolutely right.
yes, this is the correct answer. You should post this as an answer!
The question is overly simplified as indeed the volume of CO2 and H2O gas should be bigger than the volume of O2 used up during the burning. Given that the CO2 dissolves in water, and H2O can condense, the volume of gases can indeed decrease, leading to an underpressure in the bottle, so that water is pulled in (or if you prefer, pushed in by the external pressure).
As for what happens after the flame is extinguished, I believe there can be no misunderstanding that the temperature effects will dominate. The gas in the bottle will cool down, and the remainder of the water vapor will condense, both of which cause the water level to rise even further.
I should also add that pressure doesn't 'pull', it pushes. The water level rises as a result of the atmospheric pressure pressing down on the surface of the water outside the bottle.
"The amount of oxygen the candle can use is more than the CO2 it produces, so water is sucked up into the bottle from the bowl, making the water level inside the bottle slowly rise."
Not really.
The oxygen burned up by the candle is fixed to the hydrocarbons of the wax and the wick of the candle, producing carbon dioxide and water vapour. The partial pressure of the water vapour and carbon dioxide products of combustion are actually a tiny bit higher than the partial pressure of the oxygen used up, due to the higher densities of these gases, but the effect is negligible compared to temperature effects. The partial pressure from the CO2 alone should be less than the partial pressure of the oxygen before being combusted, but the partial pressure of the water vapour should make up for that, and there isn't really enough time passing here to consider the condensation of the water vapour back into liquid water.
I got the correct answer, but I had to sort of guess what you were thinking in your reasoning of the question. Afterward, I tried the experiment myself with a glass bottle and a plastic bottle. When the glass bottle is placed over the candle, at first, the water level seems to push down slightly inside of the bottle, then, almost immediately afterward, the candle begins to extinguish itself from lack of oxygen. Maybe my candle was too big in comparison with my bottle, but the result was much faster than it appears in your video. While the candle was flickering, the water level rose a little. After the candle was extinguished, the water level inside of the bottle rose significantly. That observation leads me to believe that the temperature effects dominate the behaviour. Repeating with a plastic bottle, the high water mark was even higher. My hypothesis is that the carbon monoxide from incomplete combustion may be able to breathe through the plastic bottle, which causes the flickering candle to cause more water level rise in the plastic bottle than in the glass bottle. Maybe, if this is so, the carbon monoxide can breathe through the water itself as well, leading to the behaviour shown in your graphic.
Log in to reply
you should post your own answer...
I think the key here might be, that a burning candle is actually a crappy burning process. Most of the oxidation happens to the wax and only a fraction burns to CO2 and water (which is also evident by the yellow flame and the smoke). Therefore his statement might be right.
We must also not forget that there will be unburnt wax vapours released as soon as the candle is extinguished. And the temperature drop takes a finite time. So the water level actually drops initially.
Log in to reply
Why?
Chemistry is never really that simple as to Rohit Gupta's solution...
Log in to reply
Sometimes in high schools, you wish we lived in a simple world...
That is what I assumed. I didn't take the temperature change into account.
Great. Love it.
Not true, when the candle was lit, the temperature of the started to rise. Say from room temperature (294K) to 36°C (319K); d(T)=15K. To claim that it was immediately high is not true.
Wouldn't it be better if u made it understand through only the context of pressure, as the candle burns more gas than it produces at first, pressure goes down pulling up water. When the candle extinguishes, the volume contracts as it cools, hence pulling up water further?
After the candle extinguished, the temperature of the chamber will goes colder, so the volume will be smaller leads to the rise of the water level
The volume of the gas will decrease.
How do you account for the water rise before the flame goes out?
Log in to reply
The question asks for after there is no any oxygen, so your condition is not discussed.
Carbon dioxide is about 1.5 times heavier than air or O2. The water rises as the pressure from CO2 is greater. When there is no any oxygen co2 gets the upper hand and the water rises a bit more further
If CO2 causes more pressure against the water shouldn't the water be pushed down a little?
This is just wrong
how does the water level rise if CO2 creates pressure in the bottle?
Ideal Gas Law: PV = nRT => PV/T = nR
R is a constant and n is the number of moles of gas. O2 is consumed at the same rate as CO2 is produced: there is no change in the amount of moles in the gas.
Log in to reply
I do not think O2 is consumed at the same rate as CO2 is produced. Combustion reactions such as this one: CH4 + 2O2 —> CO2 + 2H2O have Oxygen consumed at twice the rate as CO2 is produced. However, this does not include the water vapor produced, so does the number of moles of gas stays the same?
Delete this
The candle burns more gas than it produces, so the pressure goes down inside the bottle. Because of this, water is forced into the jar by the external air pressure which is higher. Once the candle extinguishes, the gas inside the jar cools. When it cools, it contracts, thereby reducing the pressure further. With the external pressure higher, more water is forced into the jar.
The candle doesn't burn gas; it's burning carbonhydrates in combination with O2. As much CO2 is produced as O2 is consumed. The volume of gas based on the changing levels of CO2/O2 is staying the same. While the candle is burning air is heating and expanding, so if temperature inside the bottle is a factor the water level inside the bottle should have lowered. And only a while after the flame went out the water should have been rising. This is clearly not the case, almost immediately the water starts rising! The answer is that water is produced in gaseous form (due to high temperature at combustion) and condenses as it cools. Reducing in volume about a factor of 1000. In some experiments during the extinguishing of the flame a large amount of soot is produced wich acts as condensation cores, wich explanes the difference in rate of level rise.
The argument here uses some assumptions that aren't EXACTLY true, but are close enough that it makes the correct predictions.
This entire phenomenon is happening because there is some tiny pressure difference at each step, and that produces a force on the water. So if the process is slow enough we can say that the total pressure remains constant. (Even if it doesn't exactly, the argument is still valid because the changing of the water level is reacting specifically to keep the pressure equal. So, any tiny difference will be quickly remediated)
Once the candle finishes burning, the amount ofCO2 and air stays the same. The thing that changes now is the flame: it goes out. So the gas temperature must decrease since it is in (basically) direct contact with the surrounding air which acts like a constant temperature reservoir. Now, we think back to why the water is rising in the first place. It's trying to keep the pressure constant. So let's assume that the water level reacts appropriately just so to keep the pressure constant.
Assuming that the gas can be approximated to an ideal gas, we have PV = N k T where P is pressure, V is volume, N is the number of particles, T is the temperature, and k is the Boltzmann constant. We see that everything is being held constant except for V and T which are free to change. (Other solutions here have mentioned that N may decrease due to dissolving into the water, but that only seeks to strengthen this argument, so we don't need to assume it)
So V is directly proportional to T. T will decrease until the gas reaches thermodynamic equilibrium with the surrounding air, so V will likewise decrease. If the volume of the trapped gas decreases, since the pressure seeks to remain constant, the water will continue to rise until the temperature stabilizes.
All of you are missing the big point here! Temperature rise or drop cannot account for water level rise (while there is a flame air should expand en blow bubbles!), Gay-Lussac says that every amount of gas has the same volume at a sertain pressure and temperature (O2 is consumed at same rate as CO2 is produced, so that doesn't explain water rise as well!). The big elephant in the room is that water is produced (in gasuas form) and it condenses into a liquid (redusing volume of about 1000x). This and this only accounts for water rise.
When the candle extinguishes there is no longer any force pulling the water up, although the water is still rising because it has to decelerate to the point where it stops going upwards and starts accelerating downwards! (if it does)
The air expands when there is heat. So if you take away the oxygen, there will be no heat, and the expanded air will return to its normal state and contract. Thus creating the vacuum effect.
Once the candle goes out, the “air” inside the bottle (now depleted of O2) will cool down, reduce pressure quickly and continue to suck up a little bit more water. The plastic will expand while the candle burns and start to contract once the candle goes out, but the contraction is not enough to force the water to go out of the bottle (air’s specific heat is 0.24 cal/gm/C and plastic’s specific heat is 0.28 cal/gm/C or higher [assuming PTFE or PET bottle] ref: https://www.engineeringtoolbox.com/specific-heat-capacity-d_391.html). So air will contract “more” than the plastic bottle with net effect that the water will rise.
Temperature & volume of the air would change after candle extinguished. Just notice this truth and get the answer.
The candle going out won't substantially change that pressure system, at first glance one would say "it stays the same" however, the gasses in the bottle are hot and will cool over time, creating its own pressure force after the chemical reaction stops
It is clear that the water level will go up when the candle stops burning, because the temperature of the gas drops. What is not clear: why does the water level raise while the candle is burning?
The main byproducts of the combustion are CO 2 and H 2 O. Since wax is made about heavy carbohydrates with approximately 2 hydrogen atoms for each carbon atom, about half of the oxygen turns into CO 2 and and the other half will go into H 2 O.
One oxygen molecule produces one carbon-dioxide molecule and the number of moles in the gas does not change. Consequently the pressure does not change. Even worst: one oxygen molecule produces two H 2 O molecules and therefore the partial pressure of the oxygen that goes through that process is actually doubled !
But we all know, from observation, that the water level goes up. To me, the most likely explanation is that the H 2 O molecules quickly condense into water (mostly on the inner surface of the glass container), taking the oxygen out of the gas mixture.
When the candle was extinguished then it means that all oxygen inside the bottle was used up. Also , there is no further release of CO2. As a result , empty space inside the bottle will increase which leads to rise in the water level.
When the candle was lit, the temperature of the air was high. Now, on extinguishing, the temperature drops and so does the pressure, pulling the water up.