2,6-dibromo Acetophenone Nitrogenation
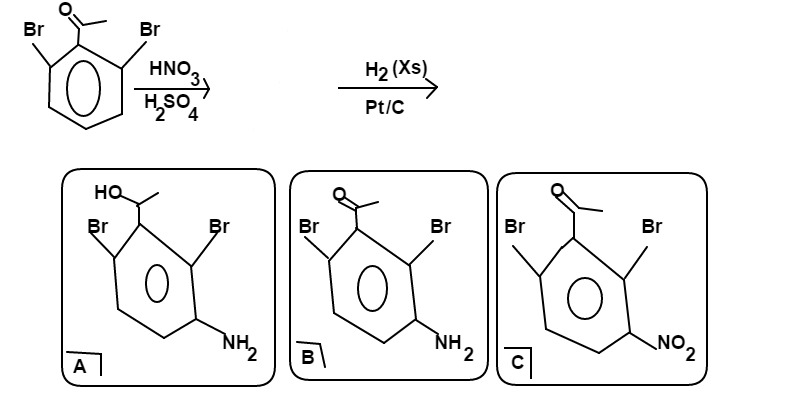 is reacted in the following order:
is reacted in the following order:
-
in .
-
in excess ( ) with .
From among choices , , and ; choose the major product of this reaction scheme. If the major product is not among the choices, choose as your answer.
David's Organic Chemistry Set
David's Physical Chemistry Set
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Both B r s will act as ortho/para directors while the a c e t y l group acts as a meta director. There are two carbons that all these groups will direct nitration to, but nitration of either will yield the same product
H 2 with a P t / C catalyst reduces the a c e t y l group to a 2 ∘ alcohol and N O 2 to N H 2 ; thus, the major product is choice A
A n s w e r → A = 2 , 4 - d i b r o m o - 3 − ( 1 - h y d r o x y e t h y l ) a n i l i n e