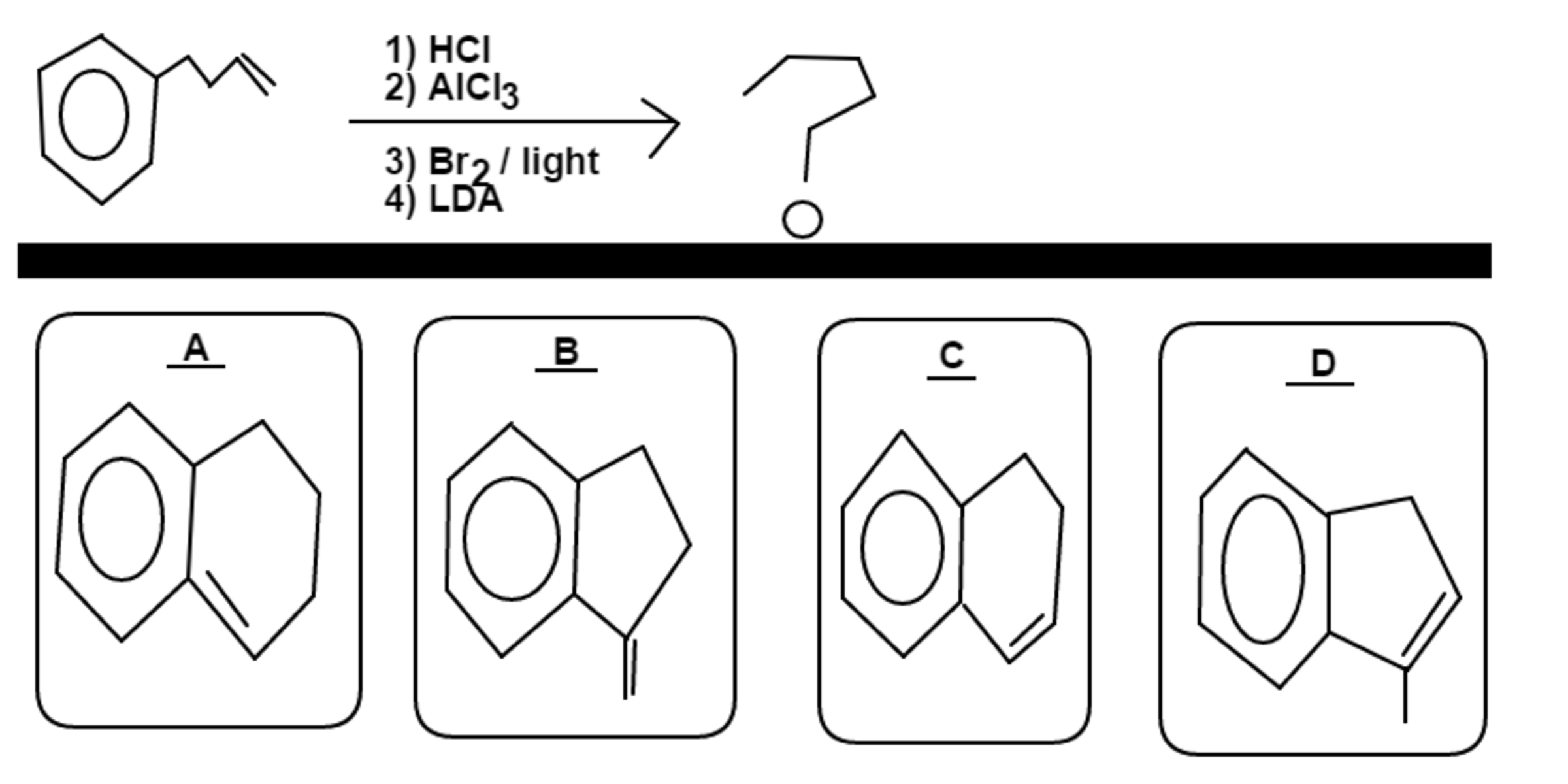
 Starting with
, you proceed to react it in the following order:
Starting with
, you proceed to react it in the following order:
-
-
-
in
-
Among choices through , choose the major product of the above series of reactions.
David's Organic Chemistry Set
David's Physical Chemistry Set
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
H C l is added to the double bond, with C l attaching to the most substituted carbon in following Markovnikov's Rule
A l C l 3 causes Friedel Craft Alkylation to occur, causing the chlorine-bonded carbon to form a ring onto benzene
B r 2 will then add to the preferred 3 ∘ carbon. Note, l i g h t is not energetic enough to add B r to an aromatic group like benzene
L D A will remove B r to form a terminal double bond that is in conjugation with b e n z e n e . The least substituted double bond is preferred because L D A is a bulky reagent
M a j o r P r o d u c t → B