Chemistry feels lonely without Organic!
An alcohol
- has 4 carbon and 1 oxygen atoms
- gives white/colourless in Victor Mayer test.
Here are the sequential reactions carried out on it.
Reactions:
- Add Cu to it and heat it to 350°C.
- Then add .
- Add PCC to it.
- Then add isopropyl Magnesium Chloride to it followed by
- Add to it.
- Now add
- Now add . Consider the stable (major ) product.
- Add Hypobromous acid to it.
- Now add aq. KOH (excess ) to it.
- Now add Methyl Magnesium Iodide to it in the presence of .
- At last add to it.
Enter your answer as the Molecular weight of the end product.
Hints and Details
- Every step of reaction will happen.
- Consider the major products,
- End product has a ketonic group.
Wanna! Try more on Organic then , Click here
Wanna! Try more on Chemistry then , Click here
The answer is 128.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
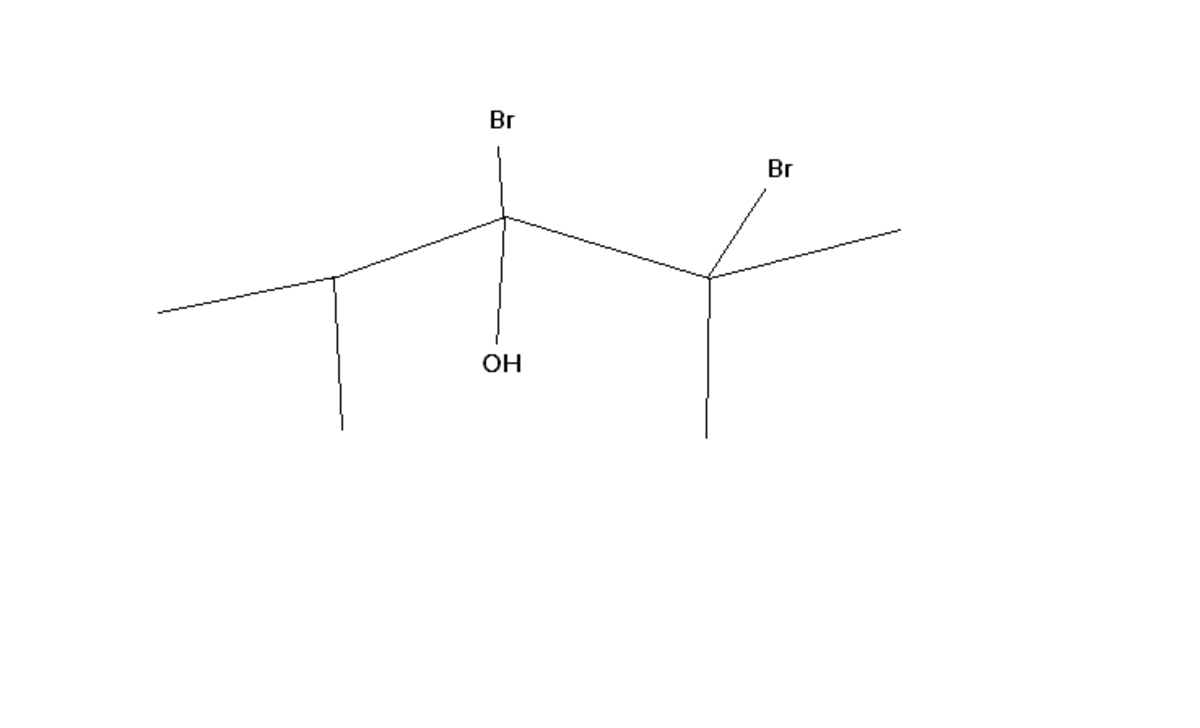
A is the end product for this question .