Chemistry isn't Mugging Up!
Chemistry
Level
3
The total number of
bonds in the following compounds is S.
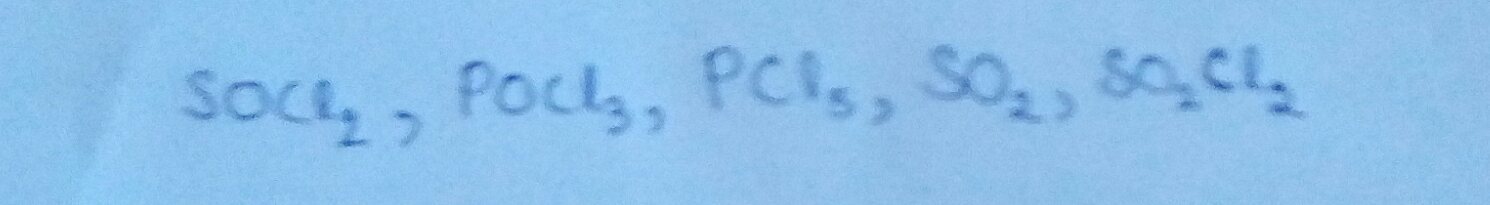
Input S+1.
The answer is 6.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
The only p π type atom in the question is O (all others are d π ), and in all cases it is doubly bonded with other atoms. Thus, we only have to find the total number of bonds consisting O , which is 6.