Christmas Brownies

In preparation for christmas dinner, Calvin was cooking macaroni and cheese in a metal pot over an electric stove set on low heat. Once it's done, he set it aside directly on the granite countertop, but forget to turn off the stove (as he's busy cooking). He then took out the brownies from the oven, which were baking in a glass tray, and set them on top of the stove (due to a lack of space).
What happened to the tray of brownies?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.

This is the exact solution: [to both Calvin and Aditya]
The first two options are very, very wrong as the "electric inductive stove" cannot transfer the heat to the brownies since the medium of the tray is glass {glass is not very electro-conductive}, so you will never see the brownies being burnt.
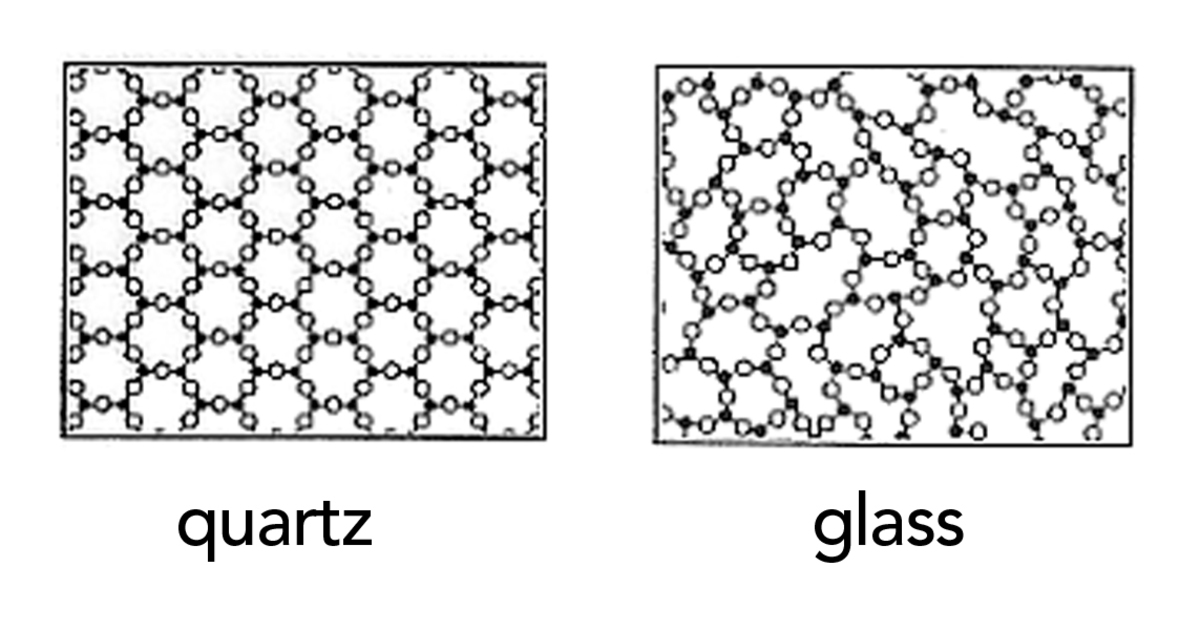
However, the main reason the glass tray exploded in this scenario is because the glass is an "amorphous solid" (can't find a proper term). Therefore, the glass molecules are "jiggling" as it is cooking in the oven, making the structure unstable. When it is placed on a metal or a glass cook top, the heat must be dissipated, but since the glass in the oven tray is also not heat conductive, it dissipates the heat very rapidly from the air that it soon becomes extremely brittle and breaks due to the 'thermal stress' [cudos to Berkal Tiliksew]. This is why we need a towel to dissipate the heat so it cools down at a lower rate.
Now Calvin is definitely lamenting on the fact glass shards are in your favourite brownies.