Court of Owls
After locking up Scarecrow in Arkham Asylum, Batman returns to Mr.Freeze's lab to thank him.
He finds Mr.Freeze unconscious and looks around his laboratory to investigate. He finds a message on the wall.
"The court of owls awaits you"
Batman tries to trace the source of the message, but must answer this question to decode the encryption.
"What is the bond order of the compound whose chemical formula is the same as the first two letters of the word co urt
(This question must be answered using ideas from molecular orbital theory)
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
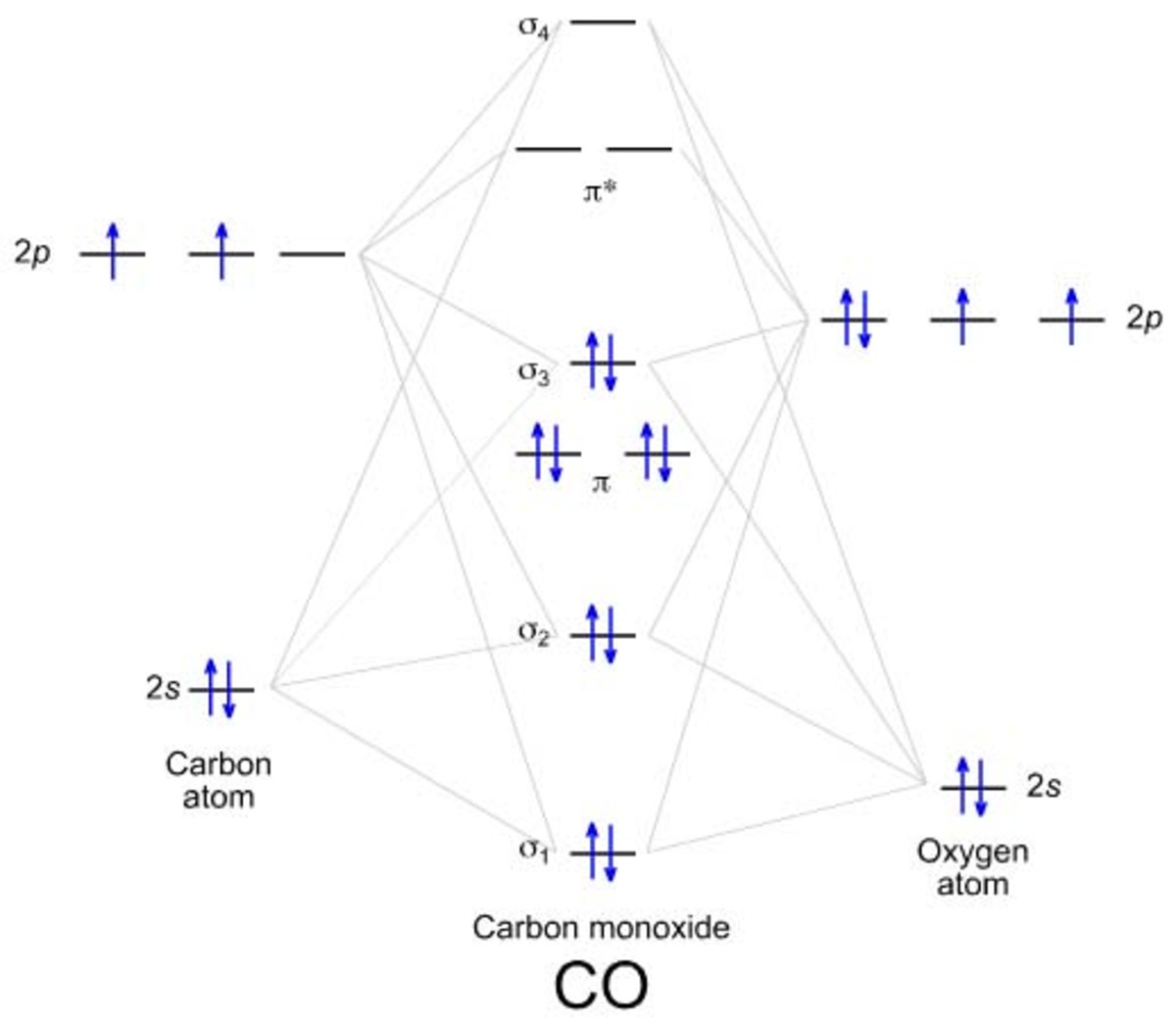
Else write MO configuration of CO noting that it has 14 electrons... ( σ 1 s ) 2 ( σ ∗ 1 s ) 2 ( σ 2 s ) 2 ( σ ∗ 2 s ) 2 ( π 2 p x ) 2 ( π 2 p y ) 2 ( σ 2 p z ) 2 Bond Order = 1 0 − 4 / 2 = 3 according to MO theory.