Creating and testing unknown gas
People create a gas
in order to test its properties. Therefore, they make a setup according to the picture below:
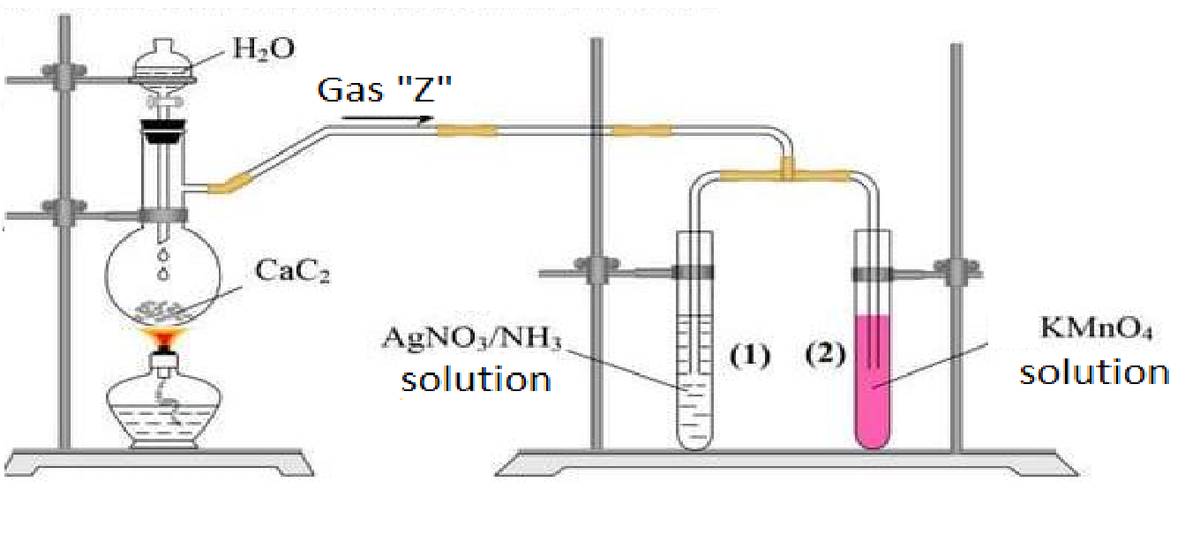 After waiting for all the chemical reactions to be completely done, which of the following best describes what happened?
After waiting for all the chemical reactions to be completely done, which of the following best describes what happened?
Bonus: Write all the chemical reactions which happened.
Go to Problem: Identify if you know what is .
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
In the test tube (1), silver acetylide( A g 2 C 2 ) is produced. Also, in the test tube (2), manganese dioxide( M n O 2 ) is produced, which is dark brown.