This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
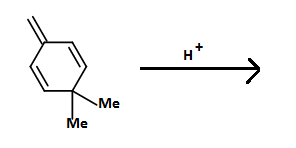
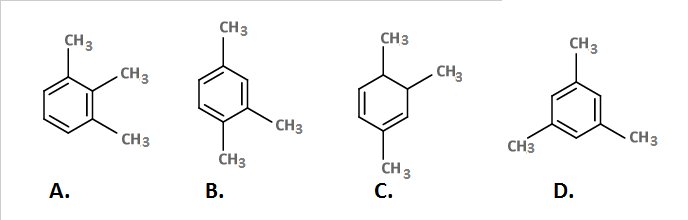
First the H+ attacks the pi-bond outside the ring and forms a carbocation which resonates to the alpha position of the dimethyl groups by conjugation. One of the methyl groups gets shifted to this position by rearrangement and a proton is eliminated forming a pi-bond that aromaticizes the ring. The final product is 1,2, 4- trimethylbenzene.