Density of the air
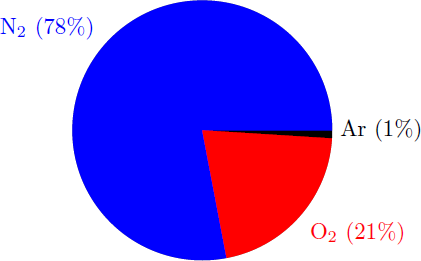
Air, if the trace gases are neglected, consists essentially of 78% nitrogen, 21% oxygen, and 1% argon (in volume percent).
What is the density of the air when 1 mole of air occupies a volume of liters (standard conditions)?
Give the result in units of and round to the first decimal place.
Note: Air is composed almost entirely of the isotopes and
The answer is 1.3.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
The molecules/atoms each have the molar masses M N 2 = 2 8 g / mol , M O 2 = 3 2 g / mol , and M Ar = 4 0 g / mol , so that the density results to ρ = V m ∑ i c i M i = 2 2 . 4 0 . 7 8 ⋅ 2 8 + 0 . 2 1 ⋅ 3 2 + 0 . 0 1 ⋅ 4 0 l g ≈ 1 . 3 m 3 kg