Directing Nitration
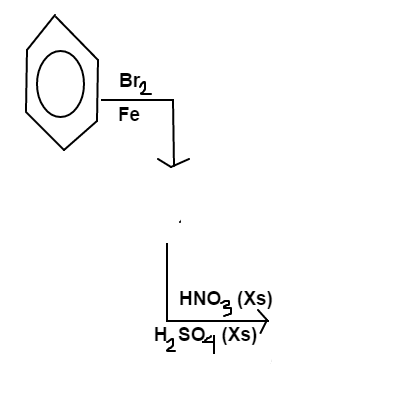 Benzene is added to a solution of
and
. The major product is then added to a solution with an excess (
) of
and
.
Benzene is added to a solution of
and
. The major product is then added to a solution with an excess (
) of
and
.
Determine the total number of major products formed after excess nitration.
Note: Be aware that some groups on a benzene are directing while others direct .
David's Organic Chemistry Set
David's Physical Chemistry Set
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
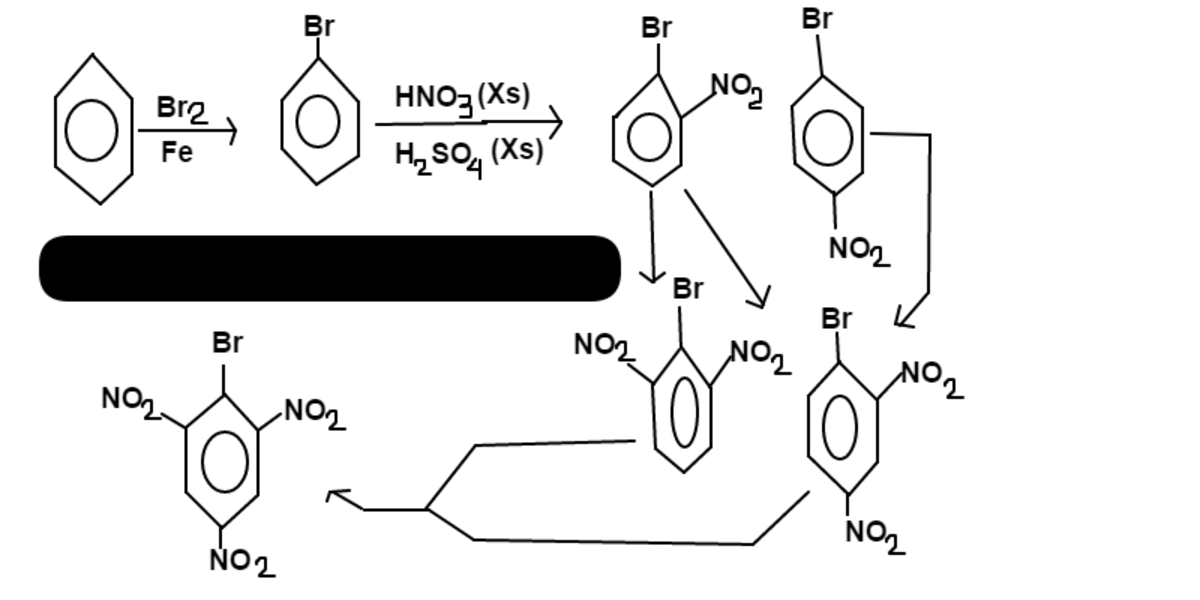 Once
bromo benzene
is formed, the bromine will begin adding
nitro
groups ortho and para to it. From then on, bromine will continue to add
nitro
groups ortho and para to itself while the
nitros
will assist in adding to spots that are, at the same time, meta to themselves.
There is only
1
major product formed:
2,4,6-trinitro phenylbromide
.
Once
bromo benzene
is formed, the bromine will begin adding
nitro
groups ortho and para to it. From then on, bromine will continue to add
nitro
groups ortho and para to itself while the
nitros
will assist in adding to spots that are, at the same time, meta to themselves.
There is only
1
major product formed:
2,4,6-trinitro phenylbromide
.