Do Golem breathe?
"I have seen a medicine, that's able to breathe life into stone . . ." .
 (Image from DeviantArt)
(Image from DeviantArt)
In 2011, Russian researchers investigated a compound that appears to have tasted the medicine that Lafew describes in Shakespeare's play All's Well That Ends Well .
Consider a compound B of d-metal A whose chemical formula is . When 0.948356g of this substance reacts with 0.23787g of and an excess of fluorine gas, it is observed that 1.388106416g of a red solid C is formed and 0.3334g of chlorine gas is evolved.
A forms a chelate complex with two moles of an organic compound D which is a symmetric diketone whose formula is . This complex is dissolved in toluene and mixed with another organic compound W to form a large solvate complex Q . The process of producing W from precursor (shown below) proved to be inefficient.
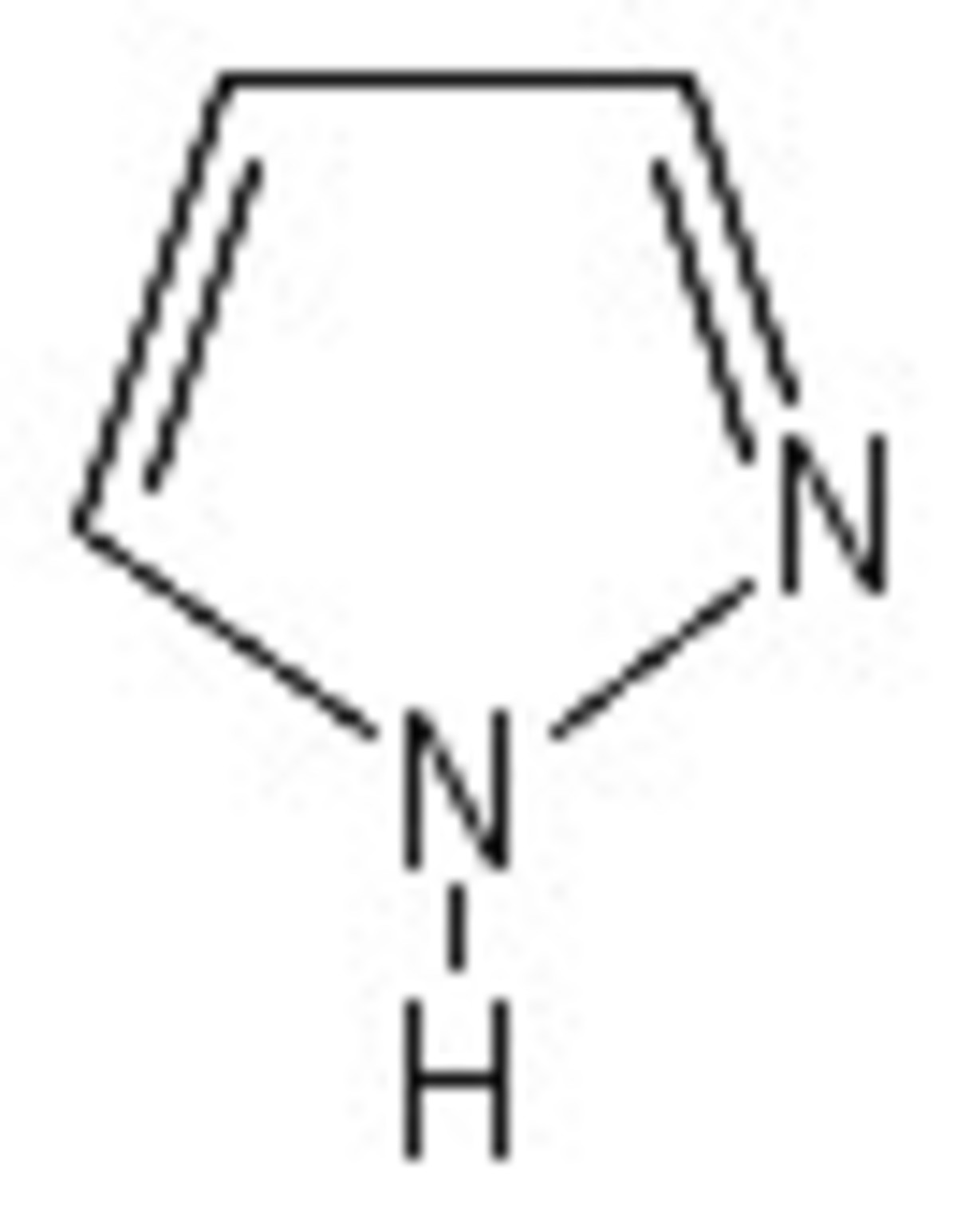
To overcome this, the following sequence was introduced. We start with a compound T that is an acetal whose H-NMR contains 4 distinct signals and its intensities are in the ratio 1:1:4:6.
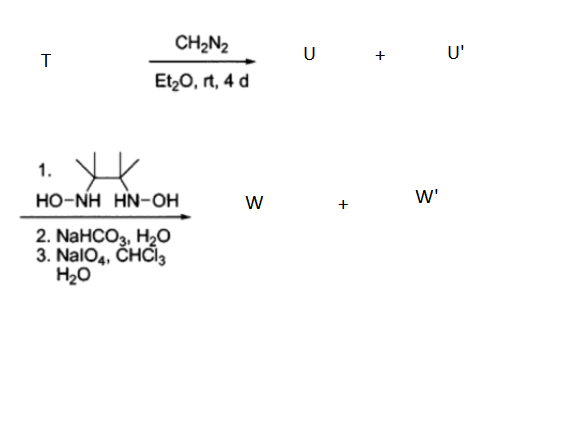
W is radical species of the formula .
The final product mix contains regio-isomers that can be easily separated. We distinguish the isomers using a H-NMR machine that can detect coupling no greater than 3J values. W observes coupling at regions greater than 7.9 ppm, while W' observes coupling in the 5.9 - 7.2 ppm range.
The solvate complex forms many different crystals, one of these crystals contain a long chain of repeating units and we call this unit G . G can be considered to be an octahedral complex and at a transition temperature of 150K it changes from a trans form to a pseudo-cis form in a reversible manner without disturbing the integrity of the chain.
Let n denote the number of pseudo-cis units, it is given by the following statistical equation :
for all temperatures T less than 150K
When many units G morph into the pseudo-cis configuration the crystal shrinks in volume. The ratio of volume at T and the volume at 150K be governed by the empirical formula :
We now define certain quantities:
is the number of rings in W
is the valency of A in compound C
is the ratio between volumes at T = 120K (taken up to two decimal places)
Calculate the value of
Bonus:
- Deduce the formula of compounds A - C
- Deduce the structure of compounds T - W
- Deduce the structure of G in trans configuration
- If T is varied periodically, what motion does the crystal mimic?
The answer is 105.
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
0 solutions
No explanations have been posted yet. Check back later!