Dynamic equilibrium
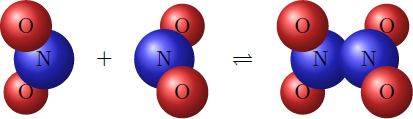
The gas nitrogen dioxide ( N O X 2 ) reacts in an exothermic reaction to the gas dinitrogen tetraoxide ( N X 2 O X 4 ) : 2 N O X 2 ( g ) N X 2 O X 4 ( g ) + h e a t . Since both forward reaction and reverse reaction take place, a dynamic equilibrium forms after some time. The equilibrium is determined by the law of mass action K = [ N O X 2 ] 2 [ N X 2 O X 4 ] , where K is the equilibrium constant. (In the gas phase, square brackets indicate partial pressure.)
How can the reaction be shifted to the product side, so that the equilibrium constant K is increased?
This section requires Javascript.
You are seeing this because something didn't load right. We suggest you, (a) try
refreshing the page, (b) enabling javascript if it is disabled on your browser and,
finally, (c)
loading the
non-javascript version of this page
. We're sorry about the hassle.
Only change of temperature can change the equilibrium constant.
More precisely, if a reaction is an endothermic reaction, then K is increased with the temperature increasing . If a reaction is an exothermic reaction, K is increased with temperature decreasing .
The reaction above is an exothermic reaction, so we can increase its K by the only way——decreasing temperature.